Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 51401. |
20 mL of a solution containing ferrous sulphate and ferric sulphate acidified with H_(2)SO_(4) is reduced by metallic zinc. The solution required 27.4 mL of 0.1 N solution of K_(2)Cr_(2)O_(7) for oxidation. However before reduction with zinc, 20 mL of same solution required 17.96 mL of same K_(2)Cr_(2)O_(7). Calculate the mass of FeSO_(4) " and " Fe_(2)(SO_(4))_(3) per litre of the solution. |
|
Answer» Titration before reduction gives only `FeSO_(4)`. Milli EQUIV. of `K_(2)Cr_(2)O_(7)` after reduction `=27.4xx0.1=2.740` Milli equiv. of `K_(2)Cr_(2)O_(7)` before reduction `=17.96xx0.1=1.796` Milli equiv. of `Fe_(2)(SO_(4))_(3)` in 20 mL =0.944 Milli equiv. of `FeSO_(4)` in 20 mL =1.796 `therefore FeSO_(4)(gL^(-1))=(1.796)/(1000)xx"Eq. mass"xx40` `=(1.796)/(1000)xx152xx40=10.92` `Fe_(2)(SO_(4))_(3)(gL^(-1))=(0.944)/(1000)xx"Eq. mass"xx40` `=(0.944)/(1000)xx200xx40=7.55` |
|
| 51402. |
20 mL of a solution containing 0.2 g of impure sample of H_(2)O_(2) reacts with 0.316 g of KMnO_(4) (acidic). Calculate : (a) Purity of H_(2)O_(2) (b) Volume of dry O_(2) evolved at 27^(@)C and 750 mm pressure. |
|
Answer» |
|
| 51403. |
20 ml of a hydro carbon requires 100 ml of oxygeb for complete combustion. In this reaction 60 ml of carbondioxide is produced. The formula of hydro carbon is |
|
Answer» `C_(2)H_(4)` |
|
| 51404. |
20 ml of 0.5 N HCl and 35 ml of 0.1 N NaOH are mixed . The resulting solution will |
|
Answer» be neutral Hence, solution will be ACIDIC and it will turn methyl orange red. |
|
| 51405. |
20 ml of 0.4 M H_(2),SO_(4) , and 80 ml of 0.2 M NaOH are mixed. Then the p^(H)of the resulting solution is |
|
Answer» `7` ` V_a = 20 ml ,N_b= 0.2 N, V_b = 80 ml ` ` N_aV_a = 0.8 xx 20 = 16 ` meq ` N_b V_b = 0.2 xx 8 0 = 16` meq ` therefore N_aV_a = N_bV_b rArr pH = 7 ?` |
|
| 51406. |
20 ml of 0.1 M NH, solution is titrated with 0.025M HCI solution. What is the pH of the reaction mixture at equivalence point at 25^(@)C ? (K_(b) "of " NH_(3) "is " 2 xx 10^(-6)). |
|
Answer» ` 0.1 xx 20 = 0.025 xx V_b`` 0.1 xx 20 = 0.025 xx V_b rArr V_b = 80 ml` no . OFM moles of formed `NH_4Cl =2` `M _(NH_4Cl ) = ( 2XX 10 ^(_3))/( 100 ) xx 1000 = 0.02M` ` PH =7 - ((6- log 2)/(2)) -(1)/(2) ( Log 2- 2 ) rArr pH= 5` |
|
| 51407. |
20 ml of 0.1 M FeC_(2)O_(4) solution is titrated with 0.1 M KMnO_(4) is acidic medium. Volume of KMnO_(4) solution required to oxidise FeC_(4)O_(4) completely is |
|
Answer» 20 ML `Vxx0.1xx5 =20xx0.1xx3` `V=12ml` |
|
| 51408. |
20 mL of 0.1 M H_(3)BO_(3) solution on complete neutralisation requires x mL of 0.05 M NaOH solution. The value of x will be : |
|
Answer» Solution :Boric acid is monobasic acid. `H_(3)BO_(3)+NAOH to Na[B(OH)_(4)]` `(M_(1)V_(1))/(n_(1))=(M_(2)V_(2))/(n_(2))` `(0.1xx20)/(1)=(0.05xx x)/(1)` x=40 mL |
|
| 51409. |
20 mL of 0.1 M acetic acid is mixed with 50 mL of potassium acetate. K_(a) of acetic acid = 1.8 xx 10^(-5) at 27^(circ)C. Calculate concentration of potassium acetate if pH of the mixture is 4.8. |
|
Answer» 0.1 M |
|
| 51410. |
20 ml H_2O_2is added to excess of KI in acidic medium. The liberated I_2 required 10 ml of IM hypo. The molarity of H_2O_2is |
|
Answer» 0.5M |
|
| 51411. |
20 mL N/2 HCI, 60 mL N/10 H_2SO_4 and 150 mL N/5 HNO_3 are mixed. Calculate the normality of the mixture of acids in solution. |
|
Answer» |
|
| 51412. |
20 mg of K^(o+) ions are present in 1 L of aqueous solution. Density of the solution is 0.8 mL^(-1). What is the concentration of K^(o+) ions in ppm? |
|
Answer» Solution :Mass of `K^(o+)` ions `(W_(2)) = 20 MG = 20 xx 10^(-3) G` Mass of solution `= V_(sol) xx d_(sol) = 100 mL xx 0.8 g mL^(-1) = 800 g` `ppm = (W_(2) xx 10^(6))/(W_(sol)) = (20 xx 10^(-3) xx 10^(6))/(800) = 25 ppm` |
|
| 51413. |
20 "litre" of air containing CO_(2) at STP passed through 100 mL of 0.12 N solution of Ca(OH)_(2). The filtrate obtained after the reaction required 50 mL of a solution of HCl of specific gravity 1.25 g mL^(-1) containing 0.35% by weight of acid. Find the amount of CO_(2) present in the volume of air as well as thepercentage by volume of CO_(2) in air. |
|
Answer» |
|
| 51414. |
20 gm solute is dissolved in 200 g water. Find %w/w |
|
Answer» 10 `= 9.091` |
|
| 51415. |
20 gm of sample Ba(OH)_(2) is dissolved in 10 ml of 0.5 N HCl solution, The excess of HCl was titrated with 0.2 NaOH. The volume of NaOh used was 10 mol. Calculate the % of Ba(OH)_(2) in the sample. |
|
Answer» `1.5%` Milli eq of NaOH consumed = milli eq. of HCl in EXCESS = `10xx0.2-2` `THEREFORE` m.eq of HCl consumed = milli eq of `Ba(OH)_(2)=5-2=3` Thus, equire of `Ba(OH)_(2)=3XX10^(-3)` Mass of `Ba(OH)_(2)` = Equivalents `xx` Eq. wt `=3xx10^(-3)xx(171//2)` `=0.2565gm` `therefore % Ba(OH)_(2)=(0.2565)/(20)xx100=1.28%` |
|
| 51416. |
2.0 g sample of NaCN is dissolved in 50 " mL of " 0.3 M mild alkaline KMnO_(4) and heated strongly to convert all the CN^(ɵ) to OCN^(ɵ). The solution after acidification with H_(2)SO_(4) requries 500 " mL of " 0.05 M FeSO_(4) Calculatethe percentage purity of NaCN in the sample. |
|
Answer» Solution :(a). `undersetunderset(x=2)(x-3=-1)(Coverset(+2)(N^(ɵ)))toundersetunderset(x=4)(x-3-2=-1)(OCoverset(+4)(N^(ɵ)))+2E^(-)(n=2)` (b). `3e^(-)+undersetunderset(x=7)(x-8=-1)(MnO_(4)^(ɵ))toundersetunderset(x=3)(x-4=0)(MnO_(2))` (c). `5e^+MnO_(4)^(ɵ)toMn^(2+)` `(n=5` in acidic medium) (d). `Fe^(2+)toFe^(3+)+2e^(-)(n=1)` `m" Eq of "KMnO_(4)` added in basic medium`=50xx0.3` (n-factor`=3`) `=45.0` `m" Eq of "KMnO_(4)` in acidic medium `(n=5)` LEFT after reaction with `NaCN=m" Eq of "FeSO_(4)` used `=500xx0.5xx1` (n-factor`=1`) `=25.0` `m" Eq of "KMnO_(4)` (n-factor`=3`) left`=(25xx3)/(5)=15` `m" Eq of "NaCN` in sample `=m" Eq of "KMnO_(4)` added `-m" Eq of "KMnO_(4)` left `=45-15=30` `THEREFORE(Weight)/(Ew of NaCN)xx10^(3)=30` `[Ew of NaCN=(49)/(2)` (n factor`=2`)] `(W)/((49)/(2))xx10^(3)=30` `W_(NaCN)=0.735g`. `% of NaCN=(0.735)/(2.0)xx100=36.75%` |
|
| 51417. |
20 gm of CaCO_(3)is allowed to dissociate in a 5.6 litres container at 819^(@) C. If 50% of CaCO_(3) is dissocitated at equilibrium the 'K_(p)' value is |
|
Answer» <P>5 atm  Molar con. at EQULIBRIUM - `(X)/(5.6)` Given x=50% of 0.2 =0.1, `K_(c)=(CO_(2)]=(x)/(5.6)=(0.1)/(5.6)` but `K_(P)=K_(c)(RT)^(Delta N)=(0.1)/(5.6) xx (0.0821 xx 1092)^(1)=1.6` atm |
|
| 51418. |
20 g of sucrose solution in one litre is isotonic with a solution of boric acid containing 1.63 g of boric acid in 450 ml. Find the molecular weight of boric acid. |
|
Answer» |
|
| 51419. |
2.0 g of a sample containing NaCl, NaBr and some inert impurity is dissolved in enoughwater and treated with excess of AgNO_(3) solution. A 3.0g of precipitate was formed. Precipitate on shaking with aqueous NaBr gains 0.76 g of weight. Determine mass percentage of NaCl in the original sample. |
|
Answer» |
|
| 51420. |
20 dm^(3) of of SO_(2) diffuse through a porous partion in 60 s. What volume of will diffuse under similar conditions in 30s ? |
|
Answer» Solution :`r_(SO_(2))=(20 dm^(3))/(60 s)=(1)/(2) dm^(3)s^(-1),r_(O_(2))=(v dm^(3))/(30 s)=(v)/(30)dm^(3)s^(-1)` Appying GRAHAM's law of diffusion, `(r_(O_(2)))/(r_(SO_(2)))=sqrt((M_(SO_(2)))/(M_(O_(2)))), i.e., (v//30)/(1//3)=sqrt((64)/(32))"or" (v)/(10)=sqrt(2)=1.414 "or" v=14.14 dm^(3)` |
|
| 51421. |
20 dm^3 of an unknown gas diffuse through a porous partition in 60 s, whereas 14.1 dm^3 of O_2 under similar conditions diffuse in 30 s. What is the molecular mass of the gas ? |
|
Answer» |
|
| 51422. |
20 cc of 'x' M HCl is exactly neutralised by 40 cc of 0.05 M NaOH . The pH of HCl solution is |
|
Answer» `1.0` |
|
| 51423. |
20 calory heat is needed to increase the temperature from 25°C to 30°C of Al metal piece having 15 gram weight. Final the heat capacity, specific heat capacity and molar heat capacity for the Al piece. (Al = 27 gram/mole) |
|
Answer» SOLUTION :HEAT CAPACITY `= 4.0 "calory"//^(@) C` Specific heat capacity `=0.266 "calory"//^(@) C`, MOLAR heat capacity `= 7.2 "calory"//^(@) C` |
|
| 51424. |
2 xx 10^(8) atoms of carbon are arranged side by side. Calculate the radius of carbon atom if the length of this arrangement is 3.0 cm |
|
Answer» Solution :Total length = 3.0 cm Total number of ATOMS ALONG the length `= 2 xx 10^(8)` `:.` DIAMETER of each atom `= (3.0 cm)/(2 xx 10^(8)) = 1.5 xx 10^(-8) cm` `:.` RADIUS of the atom `= (1.5 xx 10^(-8) cm)/(2) = 0.75 xx 10^(-8) cm = 0.075 xx 10^(-7) cm` `= 0.075 xx 10^(-9) m = 0.075 nm` |
|
| 51425. |
2-Phenylpropene on acidic hydration gives |
|
Answer» 2-phenyl-2-propanol |
|
| 51426. |
2-Phenylethanol may be prepared by the reaction of phenyl magnesium bromide with |
|
Answer» `HCHO` 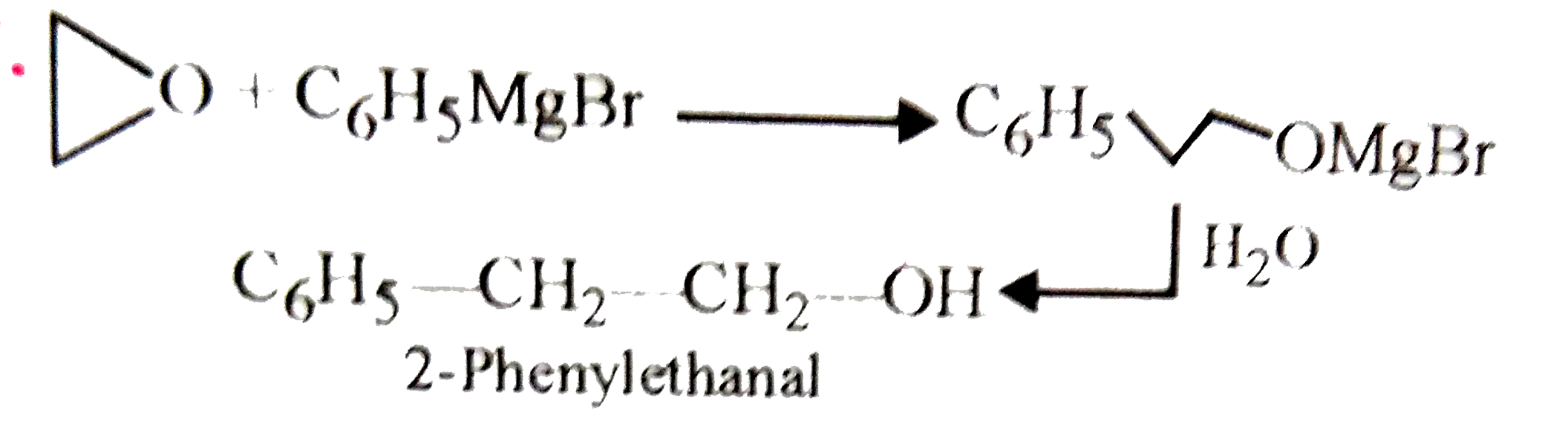
|
|
| 51427. |
2-Pentanone and 3-methyl-2-butanone are a pair of __________ isomers. |
|
Answer» Functional |
|
| 51428. |
2 N solution of sodium carbonate is equivalent to a solution of strength : |
|
Answer» 106 G PER 100 mL |
|
| 51429. |
2 N_(2) O (g) + O_(2) (g) hArr 4 No (g) , Delta H gt 0 What will be the effect on equilibrium when (i) Volume of the vessel increases ?(ii) Temperature decreases ? |
|
Answer» Solution :(i) For the given reaction, `K= [NO]^(4)/ ([N_(2)O]^(2) [O_(2)])` When volume of the vessel increases, number of moles PER UNIT volume (i.e. molar concentration) of eachreactant and productdecreases. As there are 4 concentration terms in the numerator but 3 concentration terms in the denominator, to keep K constant , the decrease in `[N_(2)O] and [O_(2)] ` should be more , i.e., equilibrium will SHIFT in the forward direction. Alternatively ,increases in volume of the vessel MEANS decrease in pressure. As forward reaction is accmpained by INCREASE in the number of moes ( i.e. increase of pressure ) , decrease in pressure will favour forward reaction ( according to Le Chatelier's principle ). (ii) As `Delta H` is + ve, i.e, reaction is endothermic , decrease of temperature will favour the direction in which heat is absorted , i.e., backward direction. |
|
| 51430. |
2 moles of N_(2)O_(4) (g) is kept in a closed container at 298 K and 1 atm pressure. It is heated to 596 K when 20% by mass of N_(@)O_(4) (g) decomposes to NO_(2). The resulting pressure is |
|
Answer» <P>2.4 ATM `=(2xx0.0821xx298)/(1)=48.9" L"` `N_(2)O_(4) HARR 2NO_(2)` `{:("Initial moles",2,0),("Moles after",2-(20)/(100)xx2,2xx0.4),("dissociation",=2-0.4,=0.8" mole"),(,=1.6"mole",):}` Tota moles present after heating`=1.6+0.8` `=2.4" moles"` `P=(nRT)/(V)=(2.4xx0.0821xx596)/(48.9)=2.4" atm"` |
|
| 51431. |
2 moles of N_(2)H_(4) loses 16 moles of electrons is being converted to a new compound x. Assuming that all of the N appears in the new compound, what is the oxidation state of N in x? |
|
Answer» `-1` `1N_(2)H_(4)rarr8e^(-)` i.e., n-factor = 8 = (`Delta` ox. No.) `xx2` implies `Delta` ox. No. = 4 Ox. No in `N_(2)H_(4)=-2` implies Final ox. No. `=-2+4=+2` |
|
| 51432. |
2 moles of KMnO_(4) present in 500 ml solution get converted into MnO_(2). The normality of KMnO_(4) solution is 3x, x = ? |
|
Answer» |
|
| 51433. |
2 moles of H atoms at NTP occupy a volume of: |
|
Answer» 11.2 LITRES |
|
| 51434. |
2 moles of gas contained in a four litre flask exerts a pressure of Il atm at 27^@C. If vander Waals parameter bis 0.05 l/mol, the value of 'a' (in atm "lt"^(2) "mol"^(-2)) is |
|
Answer» 6.46 `(P + (an^2)/(V^2)) (V - nb) = nRT` `(11 + (a XX 4)/(10)) (4 - 2 xx 0.05) = 2 xx 0.0821 xx 300` `= 11 + a/4 = 12.6 implies a = 6.4`. |
|
| 51435. |
2 moles of FeSO_(4) are oxidised by x moles of KMnO_(4) in acid medium into ferric sulphate. 3 moles of ferric oxalate are oxidised by y moles of K_(2)Cr_(2)O_(7) in acid medium. The value of (x//y) is : |
|
Answer» `6//5` |
|
| 51436. |
2 moles of an ideal gas is expanded isothermically and revrsibly from 1 litre to 10 litre. Find the enthalapy change in kJ mol^(-1). |
|
Answer» 0 |
|
| 51437. |
2 moles of an ideal gas A is taken in an adiabatic container fitted with a movable frictionless adiabatic piston always operation at 1 atm. The gas A gets converted to gas B as per the reaction : 3A(g)rarr2B(g),""DeltaH=-kJ//"mole" If 75% of A associates under the given conditions and initial temperature of teh vessel was 300 K, then calculate the final temperature of the vessel. [Given : C_(p,A(g) =20 J//K "mole" C_(p,B(g) =30J//K"mole"] |
|
Answer» |
|
| 51438. |
2 mole PCl_5 is heated in 4 L closed vessel at definite temperature. At equilibrium 55% PCl_5 remain undissociated. Find K_c. Reaction : PCl_(5(g)) hArr PCl_(3(g)) + Cl_(2(g)) |
| Answer» SOLUTION :`K_c=0.184 "MOL L"^(-1)` | |
| 51439. |
2 mole solute dissolve in 500 g solven molarity of solution is ............. |
|
Answer» 2.5 |
|
| 51440. |
2 mole of PCI_(5) is heated in a one litre vessel. If PCI_(5) dissociates to the extent of 80%, the equilibrium constant for the dissociation of PCI_(5) is |
Answer» Solution : `K_(C)=(1.6 xx 1.6)/(0.4)=6.4` |
|
| 51441. |
2 mole of ideal gasexpands isothermically and reversibally from 1 L to 10 L at 300 K. then DeltaH is : |
|
Answer» 4.98 KJ |
|
| 51442. |
2 mole of an ideal mono atomic gas undergoes a reversible process for which PV^(2)=C. The gas is expanded from initial volume of 1L to a final volume of 3L starting from initial temperature of 300K. Find DeltaH for the process |
|
Answer» `-600R` So Final temperature `T_(2)= (1 xx 300)/(3) = 100K` `Delta H = nC_(P) Delta T = 2 xx (5)/(2) R xx 200 = - 1000R` |
|
| 51443. |
2 mole of an ideal gas at 27^(@)C expands isothermally and reversibly from a volume of 4 litre to 40 litre. The work done (in kJ) by the gas is |
|
Answer» `w= -28.72kJ` |
|
| 51444. |
2 mole of an ideal gas at 27^(@)C expands isothermally and reversibly from a volume of 4 litre to 40 litre. The work done (in kJ) by the gas is : |
|
Answer» `w = - 28.72kJ` `w=-2xx8.314xx10^(-3)xx300ln.(40)/(4),` `w=-11.488 J` |
|
| 51445. |
2 mole, equimolar mixture of Na_(2)C_(2)O_(4) and H_(2)C_(2)O_(4)required V_1 Lof 0.1 M KMnO_4in acidic medium for complete oxidatiion. The same amount of the mixture required V_2 L of 0.1 M NaOH for neutralization. The ratio of V_(1) "to "V_2is x:y, then the value of x + y is (x and y are integers) |
|
Answer» Solution :No.of MOLES of `Na_2C_2O_4 =H_(2)C_(2)O_(4)=1` `4 =v_1 xx0.5 , v_1 =8L` `2 =v_2 xx0.1 impliesv_(2)=20L` `v_1:v_2=8:20` `= 2:5 =x:y , x +y=7` |
|
| 51446. |
2-methylbutane on reacting with bromine in the presence of sunlight gives mainly |
|
Answer» 1-bromo-3-methylbutane |
|
| 51447. |
2-methyl propene is isomeric with But-1-ene. They can be distinguished by: |
|
Answer» Baeyer's reagent i) `H_2C = C - underset(CH_3)underset(|)CH_3 overset(+O_3)to H_2CO + CH_3COCH_3` ii) `CH_2 = CH-CH_2-CH_3 to CH_3CH CHO + HCHO` |
|
| 51448. |
2-Methyl butane on reacting with Br_(2)in the presence of sunlight mainly gives : |
|
Answer» 1-Bromo-2-Methyl butane Ease of replacement of H-atom `3^(@) gt 2^(@) gt 1^(@)` i.e., tertiary H atom is more reactive. |
|
| 51449. |
2-Methyl butane and dimethyl propane are |
|
Answer» CHAIN isomers |
|
| 51450. |
2-Methyl-2-butene will be represented as |
|
Answer» `CH_(3)-UNDERSET(CH_(3))underset(|)"CH"-CH_(2)-CH_(3)` |
|