Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 51501. |
1.725 g of a metal carbonate is mixed with 300 mL of N/10HCI. 10 mL of N/2 sodium hydroxide were required to neutralise excess of the acid. Calculate the equivalent mass of the metal carbonate. |
|
Answer» |
|
| 51502. |
17.1 gms of Al_(2)(SO_(4))_(3) is present in 500 ml of aqueous solution. It.s concentration can be |
|
Answer» 0.1 M |
|
| 51503. |
17 gm of H_2O_2is present in 100 ml of an aqueous solution. When 10 ml of this solution is decomposed, the STP volume of oxygen obtained is |
|
Answer» 56ml |
|
| 51504. |
1.7 g of an unknown gas occupies 2.24 L at N.T.P Calculate its molecular mass. Identify the gas. |
|
Answer» 22.4 L of the gas at N.T.P has mass `= ((1.7g))/((2.24L))xx(22.4L)=17g` The molecular mass of gas `= 17 "g mol"^(-1)` The gas is AMMONIA `(NH_(3))` |
|
| 51505. |
1.7 g of AgNO_(3) dissolved in 100 g of water wire mixed with 0.585 g of NaCl dissolved in 100 g of water when 1.435 g of AgCl and 0.85 g of NaNO_(3) were formed. Justify that the data illustrates law of conservation of mass. |
|
Answer» Solution :Since water has not taken PART in the reaction, its mass (200 G) will count towards both SIDES Mass of REACTANTS `= 1.7 + 0.585 + 200 = 202.285 g` Mass of products `= 1.435 + 0.85 + 200 = 202.285 g` `:.` Law of conservation of mass is verified. |
|
| 51506. |
16gm of oxygen and 3gm of hydrogen are present in a vessel at 0^@C and 760mm of Hg pressure. Volume of the vessel is |
|
Answer» `22.4 L` |
|
| 51507. |
1.64 g of a mixture of calcium carbonate and magnesium carbonate were dissolved in 50 mL of 0.8 N hydrochloric acid. The excess of the acid required 16 mL N//4 sodium hydroxide solution for neutralisation. Find out the percentage composition of the mixture of two carbonates. |
|
Answer» |
|
| 51508. |
16.26 milligram of sample of an element X contains 1.66 xx 10^(20) atoms. What is the atomic mass of the element X ? |
|
Answer» `6.022 xx 10^(23)` atoms of X have mass `= ((6.022xx10^(23)"atoms"))/((1.66xx20^(20)"atoms"))xx(16.26mg)` `=59.0xx10^(3)mg=59.0g`. |
|
| 51509. |
1.60g of a metal were dissolved in HNO_3to prepare its nitrate. The nitrate on strong heating gives2g oxide. The equivalent weight of metal is |
|
Answer» 16 |
|
| 51510. |
1.60 g of a metal were dissolved in HNO_3 to prepare its nitrate. The nitrate was strongly heated when 2.0 g of the metal oxide was obtained. Calculate the equivalent weight of the metal. |
|
Answer» Solution :Mass of the METAL taken = 1.60 g Mass of OXIDE formed = 2.0 g `THEREFORE` Mass of oxygen that combined with 1.60 g of the metal `=2.0- 1.60 = 0.40 g` `therefore`Equivalent weight of the metal `=("Mass of the metal")/("Mass of oxygen") xx 8 = 1.60/0.40xx 8 = 32` |
|
| 51511. |
1.6 g of pyrolusite was treated with 60 mL of normal oxalic acid and some H_(2)SO_(4). The oxalic acid left undecomposed was made up to 250 mL, 25 mL of this solution required 32 mL of 0.1 N potassium permangante (KMnO_(4)). Calcualte the percentage of pure MnO_(2) in pyrolusite. |
|
Answer» |
|
| 51512. |
16 g. of oxygen occupies a volume of 22.4L at 1 atm and |
| Answer» Answer :C | |
| 51513. |
16 g of oxygen has same number of molecules as in |
|
Answer» 16 g of CO NUMBER of molecules `= ("Mass")/("Molar mass") XX "Avogadro"` No. of molecules , in 16 g oxygen `=(16)/(32) xx N_(A)=(N_(A))/(3)` In 16 g of `CO=(16)/(32)xxN_(A)=(N_(A))/(1.75)` In 28 g of `N_(2) = (28)/(28) xx N_(A)=N_(A)` In 14 g of `N_(2) = (14)/(28)xx N_(A) = (N_(A))/(2)` In 1 g of `H_(2)=(1)/(2) xx N_(A) = (N_(A))/(2)` HENCE, 16 g of `O_(2)= 14 g` of `N_(2) = 1.0 g ` of `H_(2)` |
|
| 51514. |
1.6 g of an organic compound gave 2.6 g of magnesium pyrophosphate. The percentage of phosphorus in the compound is |
|
Answer» 0.4538 |
|
| 51515. |
16 g of an ideal gas SO_(X) occupies 5.6 L at S.T.P. What is its molecular mass ? What is the value of X ? |
|
Answer» 22.4 L of the gas at S.T.P. weigh `= ((1.6g))/((5.6L))xx(22.4L)=64G` By definition, molar mass of gas `(SO_(X))=64g` `:. 32+x(16)=64or16x=64-32=32orx=2` Value of x = 2 and the gas `= SO_(2)` |
|
| 51516. |
1.6-Diethy1 cyclohexene 2. 4-Hydrocy-5-isopropy1 hept-6-yn-2-one 3. 6-Ethy1-4-isopropy1-2-methy1 decane 4. 2,-Dimethy1 cyclo pent-3-en-1-one 5. Benzonitrile 6. Bicyclo [4.1.0] hept-3-en-2-one 7.Sprio [3.5] non-5-en-2-ol 8. 1-Ethy1-2-methy1 cyclo hexane 9. 4-Bromo-2-ethy-1-methy1 cyclohexane 10. 3-Isopropy1 cyclopetane carbaldehyde 11. 4-cyclo propy1-3,6-diethy1 undecane 12. 6-(Cyclobut-2-enl1) hex-2-ene 13. 1,4-Dicyclopenty1 butane 14. Ethylidene bromide 15. Ethylene dichloride 16. trans.-1,3 -Dibromo cyclobutance 17. 2-exo-3-endo Dichloro bicylco [2.2.1] heptane 18. Benzocyclopentene 19. 2,5-Dimethy1 oxalone or 2,5-dimethy1 oxa cyclopentane 20. Methy1 viny1 carbino1 21. 3-Cyclopenteny1 ethy1 ether 22. sec-Buty1 isopropy1 ether 23. N,N-Diethy1 butan-1-amine 24. o-Toluidine 25. Isoamy1 school 26. sec-Buty1 alcohol 27. isobuty1 alcohol 28. Maleic acid 29. Caproic acid Z-Crotonalddehyde |
Answer» SOLUTION :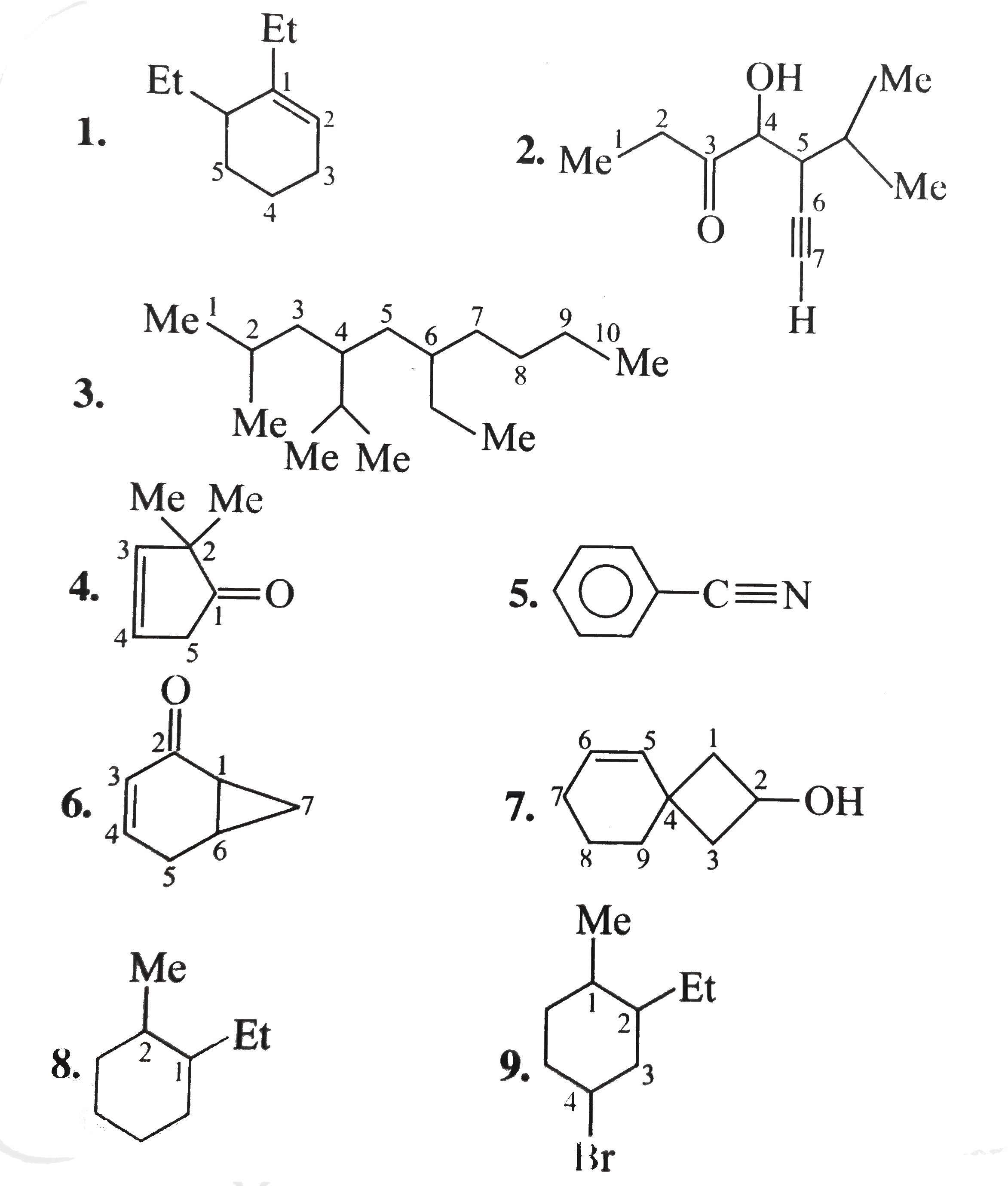 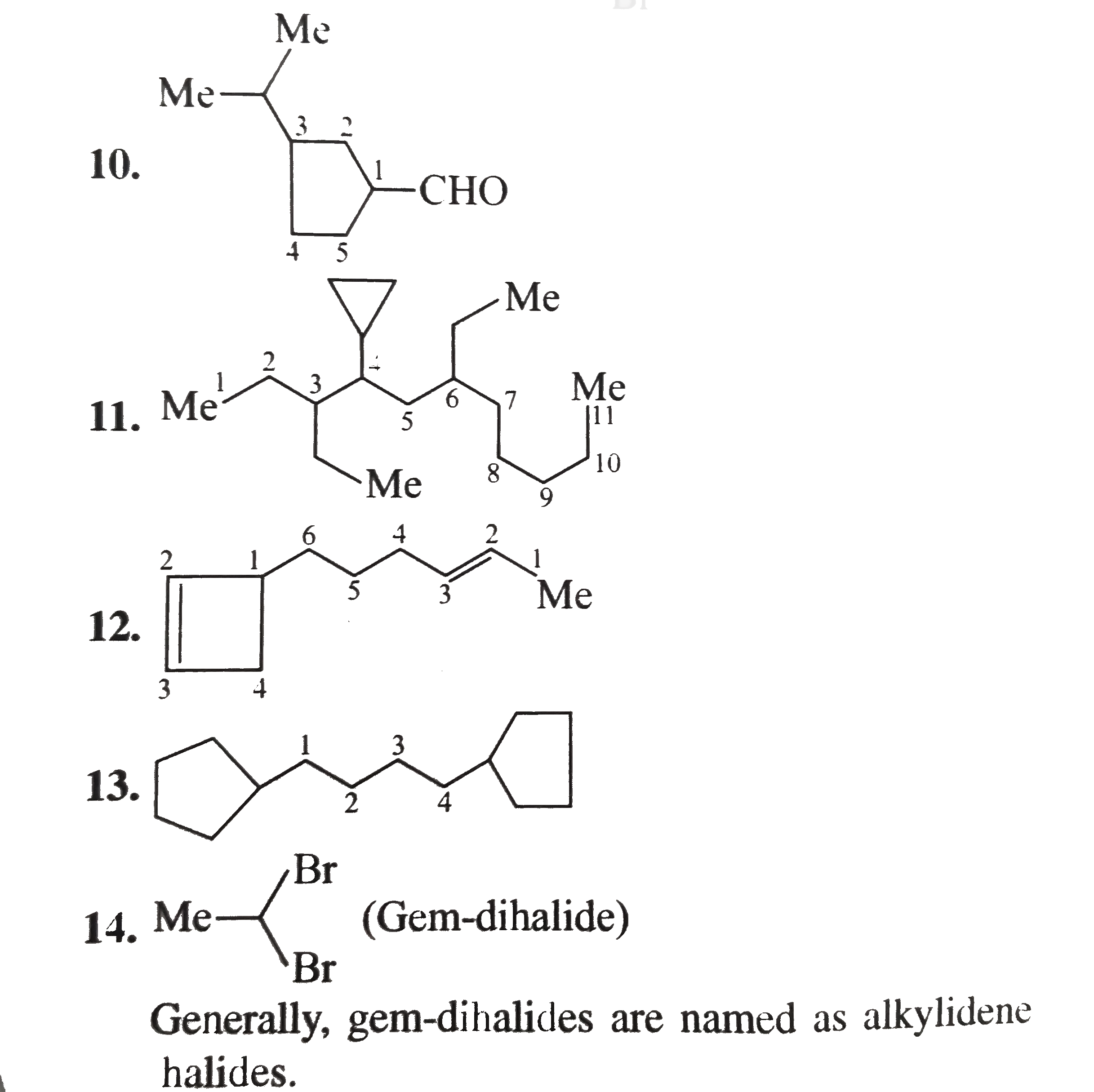 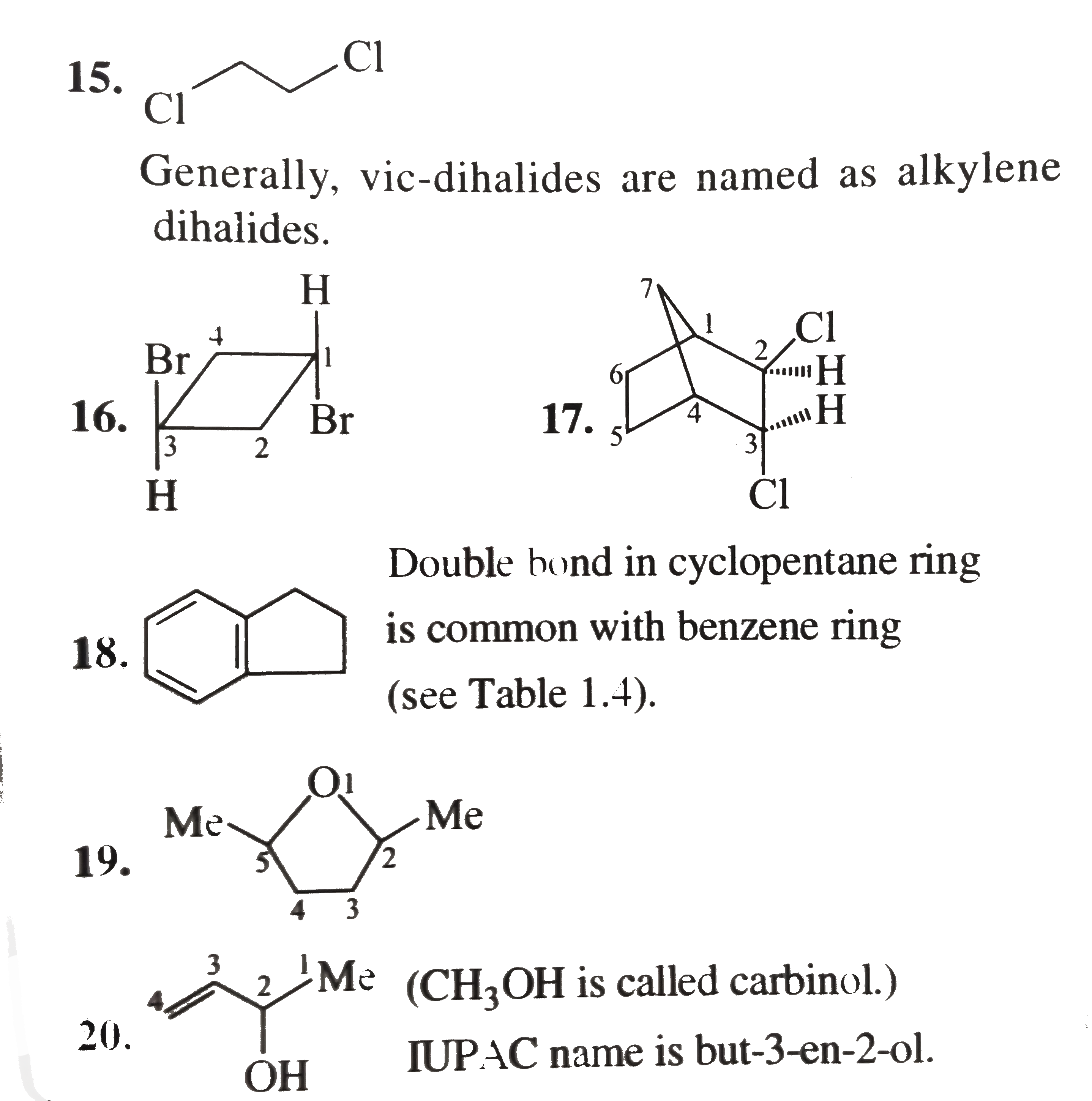 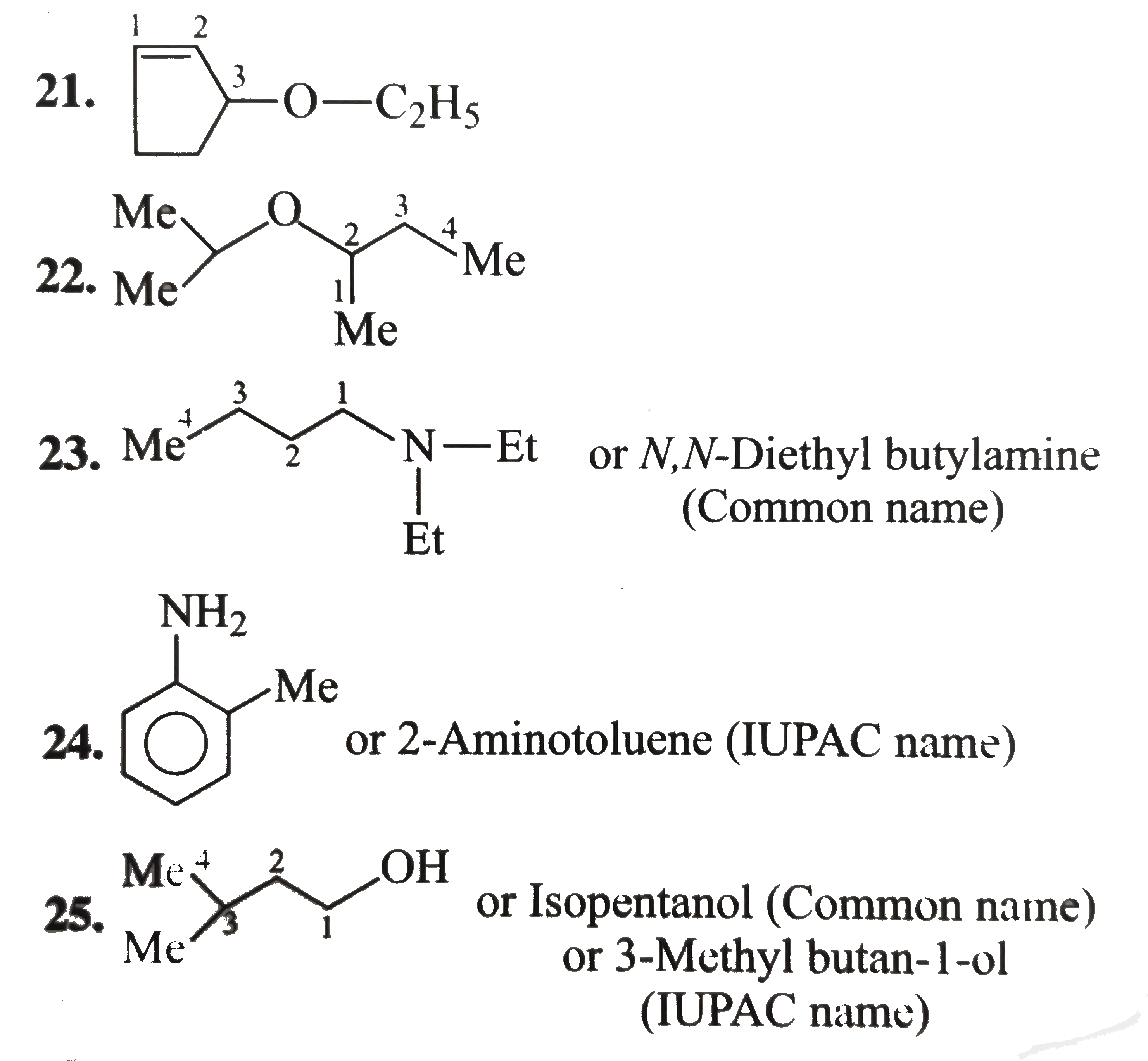 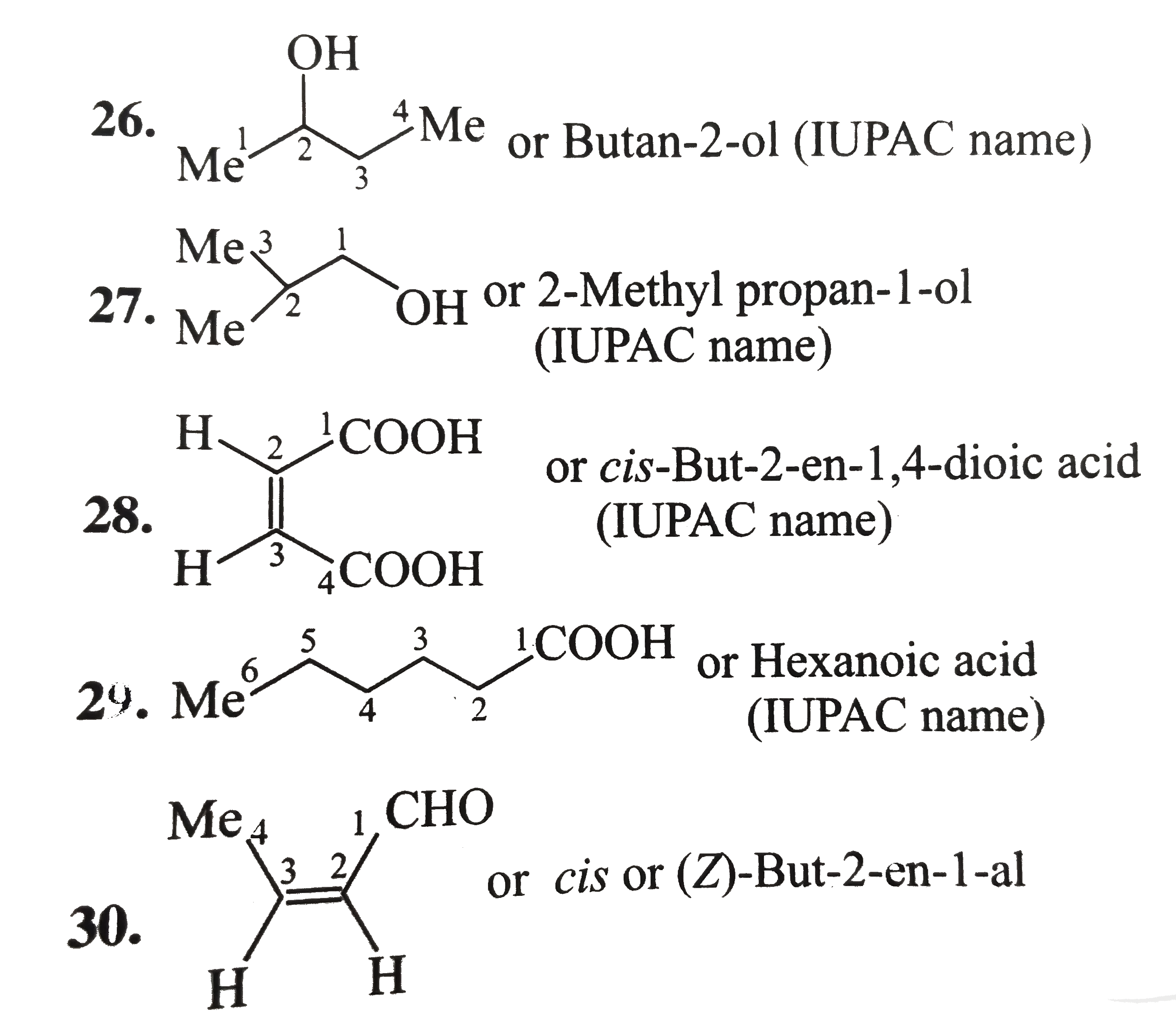
|
|
| 51517. |
15cm^(3) of hydrocarbonrequires 45cm^(3) of oxygen for complete combustion and 30cm^(3) of CO_(2) is formed. The formula of hydrocarbon is |
|
Answer» `C_(3)H_(6)` `{:("15 ml","45 ml","30 ml"),("1 ml","3 ml","2 ml"):}` `therefore x=2` `x+(y)/(4)=3 rArr (y)/(4)=1` `"hydrocarbon is "C_(2)H_(4)` |
|
| 51518. |
1.575 g of oxalic acid (COOH)_(2).xH_(2)O are dissolved in water and the volume made up to 250 mL. On titration 16.68 mL of this solution requires 25 mL of N/15 NaOH solution for complete neutralisation. Calculate the value of x. |
|
Answer» Solution :Molecular mass of oxalic ACID, `(COOH)_2 xH_2O = 24 + 62 + 2 + 18x =90 + 18x` Since oxalic acid is a dibasic acid, Eq. mass of oxalic acid `=("Molecular mass")/2` `=(90 + 8x)/2 = 45 + 9x` Normality of oxalic acid solution can be calculated from the following relation : `w=(NEV)/1000` SUBSTITUTING the values, we get `1.575 =(N xx (45 + 9x) xx 250)/1000` or `N =(1.575 xx 1000)/((45 + 9x) xx 250) = (6.3)/(45 + 9x)` According to the normality relation, `underset("oxalic acid")(N_(1)V_(1)) = underset("NaOH")(N_(2)V_(2))` `6.3/(45 + 9x) xx 16.68 = 1/15 xx 25` `45 + 9x = (6.3 xx 16.68 xx 15)/25` `=63.05 = 63` `9x = 63 - 45 = 18` or `x=18/9 =2` |
|
| 51519. |
1.53 g of a compound containing only sulphur, oxygen and chlorine after easy hydrolysis with water yielded acid products which consumed 91 " mL of " (N)/(2) sodium hydroxide for complete neutralisation in a parallel experiment, 0.4 g of the compound after hydrolysis with water, was treated with excess of BaCl_(2) solution and 0.7 g of BaSO_(4) was precipitated. What is the formula of the compound? |
|
Answer» Solution :After hydrolysis, acid products are obtained. This suggests that the substanece is an acid CHLORIDE. Reaction with `BaCl_(2)` to yield `BaSO_(4)` implies that `H_(2)SO_(4)` is one of the product. The substance is (by surmise) `SO_(2)Cl_(2)` This can be VERIFIED by the FOLLOWING way: `SO_(2)Cl_(2)(135g)=BaSO_(4)(233.4g)` `therefore0.4g-=[(233.4)/(135)xx0.4]g=0.692g` This agrees very nearly with the given DATA `=0.7g` further, 135 g of `SO_(2)Cl_(2)-=H_(2)SO_(4)+2HCl` (by hydrolysis 4 EQUIVALENTS). `1.53g-=(4)/(135)xx1.53` equivalent `=0.0453` equivalent `91 " mL of " 0.5 N NaOH-=(91)/(1000)xx0.5=0.0455` equivalent This also agrees with the given data. Therefore, the formula is `SO_(2)Cl_(2)`. |
|
| 51520. |
1.520 g of the hydroxide of a metal on ignition gave 0.995 g of oxide. The equivalent weight of metal is |
|
Answer» `1.520` `:.(1.520)/(0.995) = (x+17)/(x+8)` `:.1.520x+1.520xx8=0.995x+0.995xx17` `:.1.520x+12.160=0.995x+16.915` `:.0.525x=4.755` `:.x=(4.755)/(0.525)= 9.057` |
|
| 51521. |
1.520 g of hydroxide of a metal on ignition gave 0.995 g of oxide. The equivalent mass of metal is : |
|
Answer» 1.52 |
|
| 51522. |
15.0g of an unknown molecular material is dissolved in 450g of water. The resulting solution freezes at -0.34^(@)C. What is the molar mass of the material? K_(f) for water = 1.86K kg mol^(-1). |
|
Answer» SOLUTION :`W_(("SOLVENT"))=15.0g` ` W_(("solvent")) =450g` `Delta T_(f) = T_(1) ^(0) - T_(f) =0-(-0.34)=0.34^(@)C` `Delta T_(f)= k _(f) m` `0.34 =1.86 xx (15)/(M) xx (1000)/(450)` `M = (1.86 xx 15 xx 1000)/(0.34 xx 450) = 182. 35 g MOL^(-1).` |
|
| 51523. |
15.0 mL of 0.12 M KMnO_(4) solution are required to oxidise 20.0 mL of FeSO_(4) solution in aicdic medium what is the concentration of FeSO_(4) solution ? |
|
Answer» `2KMnO_(4)+10FeSO_(4)+8H_(2)SO_(4)rarrK_(2)SO_(4)+2MnSO_(4)+5Fe_(2)(SO_(4)^(3)+8H_(2)O)` APPLYING molarity eqautin to the above redox reaction `=(15xx0.12)/(2)KmnO_(4)=(20xxM_(1))/(10)(FeSO_(4)) or M_(1)=(15xx0.12xx10)/(2xx20)=0.45` |
|
| 51524. |
150 mL of N/10HCI are required to react completely with 1.0 g of a sample of lime stone (CaCO_3). Calculate the percentage purity of the sample. |
|
Answer» 70 Solution :Eq. mass of `CaCO_(3) = (40 + 12 + (3 xx 16))/2 = 50`.Suppose 1.0 g of the sample contains only W g of `CaCO_3`. `THEREFORE` Number of gram EQUIVALENTS of `CaCO_(3)` in 1.0 g sample `=("Mass")/("Eq. mass") = w/50` Number of gram equivalents of HCI reacted = `(N xx V)/1000` `=(1/10 xx 150)/(1000) = 15/1000` According to the law of equivalence, number of gram Eq. of `CaCO_(3) `=no. of gram Eq. of HCl `therefore w/50 = 15/1000` or `w =(50 xx 15)/1000 = 0.75 g` `therefore` Purity of the sample `=0.75/1 xx 100 = 75%` |
|
| 51525. |
150 cm^3 of a decimolar NaOH solution is diluted to 750 cm^3. Find the molarity of the diluted solution. |
|
Answer» |
|
| 51526. |
1.50 g of a metal on being heated in oxygen gives 2.15 g of its oxide. Calculate the equivalent mass of the metal. |
|
Answer» |
|
| 51527. |
150 mL M/10Ba(MnO_(4))_(2), in acidic medium can oxidize completely |
|
Answer» 150 ML 1M `Fe^(+2)` `=150 xx1/10xx10=150` |
|
| 51528. |
1*5 moles of PCl_(5)are heatedat constant temperature in a closed vessel of 4 litre capacity.At the equilibrium point, PCl_(5)" is "35 % " dissociated into " PCl_(3) and Cl_(2). Calculate the equilibrium constant. |
|
Answer» `K_(c) = (( 0* 525 // 4) (0*525//4))/((0*975 //4))=0*071` |
|
| 51529. |
15 mL 1 N H_(2) SO_(4), 25 mL of 4 N HNO_(3), and 20 mL of X M HCl were mixed and made up to 1000 mL. Prepared by dissolving 4.725 g of pure Ba(OH)_(2). 8H_(2) O in water made up to 0.25 litre. What is the molarity of HCl solution (i.e. find X) |
|
Answer» Solution :`15 mL` of `1 M H_(2) SO_(4) + 25 mL of 4 M HNO_(3) + 20 mL of X M HCL` `:. N_(1) V_(1) + N_(2) + V_(2) + N_(3) V_(3) = N_(4) V_(4)``(V_(4) = 1000 mL)` `15 xx 2 + 25 xx 4 + 20 X = N_(4) xx 1000` `:. N_(4) = ((130 + 20 X)/(1000))` mEq of mixture of acid = mEq of `Ba(OH)_(2)` `Mw of Ba (OH)_(2). 8H_(2) O = 137.4 + 34 + 18 xx 8 = 315.4` `Ew = (315)/(2) = 157.7 g` `N of Ba(OH)_(2) .8H_(2) O = (W_(2) xx 1000)/(Ew_(2) xx V_(SOL) ("in" mL))` `= (4.725 xx 1000)/(157.7 xx 250)` `0.1198 N ~~ 0.12 N` mEq of acid mix = mEq of `Ba(OH)_(2)` `20 xx N_(4) = 26 xx 0.12` `N_(4) = (26 xx 0.12)/(20) = 0.156 N` `implies (130 + 20 X)/(1000) = 0.156` `:. X = (0.156 xx 1000 - 130)/(20) = 1.3` `N` or `M HCl = 1.3` |
|
| 51530. |
1.5 gof brass containing Cu and Zn reasts with 3.0 M HNO_3 solution. The following reaction take place: Cu+HNO_3toCu^(2+)+NO_2(g)+H_2O Zn+H^(o+)+NO_(3)^(ɵ)toNH_(4)^(ɵ)+Zn^(2+)+H_(2)O (a). Calculate the percentage composition of brass. (b). How many " mL of " 3.0 HNO_3 will be required for completely reacting with 1.0 g of brass? |
|
Answer» Solution :`CutoCu^(2+)+2e^(-)` `undersetunderset(x=5)(1+x-6=0)[H^(+)+e^(-)+HNO_(3))toundersetunderset(x=4)(x-4=0)(NO_(2))+H_(2)O` `Cu+2H^(o+)+2HCO_(3)to2NO_(2)2H_(2)O+Cu^(2+)` `Cu+4HNO_(3)to2NO_(2)+2H_(2)O+Cu^(2+)+2NO_(3)^(ɵ)` or `Cu+4HNO_(3)to2NO_(2)+2H_(2)O+Cu^(2+)+2H_(2)O` 1 " mol of "`Cu=4 " mol of "HNO_(3)-=2 " mol of "NO_(2)` Mole of `NO_(2)=(PV)/(RT)=(1xx1xx0.4)/(0.082xx298)=0.0425` mole of `Cu=(0.0425)/(2)=(0.0425)/(2)xx63.5=1.3462g` Weight of `Zn=1.5-1.3462=0.1538g` `% Zn=10.25%` `% of Cu=89.75%` `[ZntoZn^(+2)+2e^(-)]xx4` `8e^(-)+undersetunderset(x=5)(x-6=-1)(NO_(3)^(ɵ))+10H^(o+)toundersetunderset(x=-3)(x+4=+1)(NH_(4)^(o+)+3H_(2)O)` or `4An+10HNO_(3)toNH_(4)^(o+)+4An^(2+)+3H_(2)O` `4Zn+10HNO_(3)toNH_(4)NO_(3)+4Zn(NO_(3))_(2)+3H_(2)O` `=3xxV` `V=0.2011 L =20.11mL` |
|
| 51531. |
15 grams of a mixture of calcium carbonate and sodium carbonate on ignition liberated a gas which has occupied 2.24L at STP. What is the weight ratio of components in the given mixture? |
|
Answer» |
|
| 51532. |
1.5 g sample of P_(2)O_(3) and some impurity was dissolved in water and warmed gentally till P_(2)O_(3) disproportionated quantitatively to PH_(3) and H_(3)PO_(4). The solutionswas then boiled to get rid off PH_(3(g)) and then cooled finally to room temperature and diluted to 100 mL. 10 mL of this solution was mixed with 20 mL of 0.3M NaOH. Now 10 mL of this solution required 3.6 mL of 0.05 M H_(2)SO_(4) for back titration. Determine % by weight of P_(2)O_(3) in sample. |
|
Answer» |
|
| 51533. |
1.5 g of sample of impure potassium dichromate was dissolved in water and made up to 500 mL solution . 25 mL of this solution required iodometrically 24 mL of a sodium thiosulphate solution. 26 mL of this sodium thisulphate solution required 25 mL of N//20 solution of pure potassium dichromate. Find the percentage purity of impure sample of potassium dichromate. |
|
Answer» Solution :NORMALITY of sodium thiosulphate solution may be determined as : `N_(1)V_(1)(Na_(2)S_(2)O_(3))=N_(2)V_(2) ("pure" K_(2)Cr_(2)O_(7))` `N_(1)xx26=25xx(1)/(20)` `N_(1)=0.048` (HYPO) The REACTION involved may be given as : `Cr_(2)O_(7)^(2-)+6O^(-)+4H^(+) to 2Cr^(3+)+3I_(2)+7H_(2)O` `3[I_(2)+2Na_(2)S_(2)O_(3) to 2NaI+Na_(2)S_(4)O_(6)]` 1 mole `K_(2)Cr_(2)O_(7)-=6 " mole" Na_(2)S_(2)O_(3)` 25 mL of solution of `K_(2)Cr_(2)O_(7)` is treated by 24 mL of 0.048 N hypo `THEREFORE 500 mL` of solution will be titrated by 480 mL of 0.048 N hypo No. of moles of hypo`=("Mass")/(M.w. (158))=(ExxNxxV)/(1000xx158)` `=(158xx0.048xx480)/(1000xx158)` =0.02304 mole No. of moles of `K_(2)Cr_(2)O_(7)=(1)/(6)`[No. of moles of hypo] `=(1)/(6)[0.02304]=3.84xx10^(-3)` Mass of `K_(2)Cr_(2)O_(7)=3.84xx10^(-3)xx294=1.12896` % purity `=(1.12896)/(1.5)xx100=75.26%` |
|
| 51534. |
15 g of ethane at 380 torr and 273^@ C occupy a volume of |
|
Answer» 11.2 L |
|
| 51535. |
1.5 g ofCdCl_(2) was found to contain 0.9 g of Cd.Calculate the atomic weight of Cd. |
|
Answer» 118 |
|
| 51536. |
1.5 g of an organic compound in a quantitative determination of phosphorus gave 2.5090 g of Mg_(2)P_(2)O_(7). Calculate the percentage weight of phosphorus. |
|
Answer» |
|
| 51537. |
15 c.c. of gaseous hydrocarbon required 45 c.c. of oxygen for complete combustion and 30 c.c. of carbondioxide is formed. The formula of the hydrocarbon is |
|
Answer» `CP_(3)H_(6)` |
|
| 51538. |
15 cc of gaseous hydrocarbon required 45 cc of oxygen for complete combusion if 30 cc of CO_(2) is formed, the formula of the gaseous compound is |
|
Answer» `C_(3)H_(6)` |
|
| 51539. |
(1)4HCl_((g)) + O_(2(g)) overset"X"to 2Cl_(2(g)) + 2H_2O_((g)) (2) 2H_2O_((aq))underset"Heat"overset(Y) to +2H_2O_((l)) + O_(2(g)) Mention the formulas of X and Y. |
|
Answer» `X=CuCl_2 , Y=NO_2` |
|
| 51540. |
14g of element X combine wilh 16 g of oxygen. On the basis of this information, which of the followings is a correct statement? |
|
Answer» The element X could have an atomic weight of 7 and its oxide is XO |
|
| 51541. |
1470 cm^3 of a gas is collected over water at 303 K and 74.4 cm of Hg. If the gas weighs 1.98 g and vapour pressure of water at 30^@C is 3.2 cm of Hg, calculate the molecular weight of the gas. |
|
Answer» `:. ""P = 74.4 - 3.2 = 71.2 " cm Hg " (71.2)/76` atm `V = 1470 cm^3 = 1470/1000 = 1.47 L, T = 303K` If the MOLECULAR weight of the gas is M, then the number of moles in 1.98 g of gas = `(1.98)/M` According to the gas equation `PV = nRT` SUBSTITUTING the values, we have `(71.2)/76 xx 1.47 = (1.98)/M xx 0.0821 xx 303` `:. ""M = 35.8` Hence, the molecular weight of the given gas is 35.8. |
|
| 51542. |
14.7 g of sulphuric acid was needed to dissolve 16.8 g of a metal. Calculate the equivalent weight of the metal and the volume of hydrogen liberated at NTP. |
| Answer» SOLUTION :56, 3.36 LITRES | |
| 51543. |
1.44 g of pure Fec_(2)O_(4) was dissolved in dilH_(2)SO_(4) and th solution diluted to 100 cm^(3) calculate the volume of 0.01 M KMnO_(4) requiredto oxidise FeC_(2)O_(4) solution completely |
|
Answer» SOLUTION :Step 1 To write the balanced equation for the redox reaction both the CATION and anioic components of `FeC_(2)O_(4)` (ferrous oxalate) i.e `Fe^(2+)` and `C_(2)O_(4)^(2-)` are oxidised by `KmnO_(4)` to `Fe^(3+)` and `CO_(2)` respectively the comlete balanced redox equation is `5 Fe^(+)+MnO_(4)^(-) +8 H^(+)rarr5 Fe^(3+) rarr 5Fe^(3+)+Mn^(2+)+4H_(2)O` `5C_(2)O_(4)^(2-)+2 MnO_(4)^(-)+16 H^(+)rarr10CO_(2)+2 Mn^(2+)+8 H_(2)O` `5FeC_(2)O_(4)+3MnO_(4)^(-)+24H^(+)rarr5Fe^(3+)+10 CO_(2)+3Mn^(2+)+12H_(2)O` Step 2 To determine the molarity of `FeC_(2)O_(4)` solution MOL wt of `FeC_(2)O_(4) =56+2xx12+4xx16=144 g` `"volume" =100 cm^(3)` `therefore "Molarity" =("weight" )/("mol.wrt")xx(1000)/("volume")=(1.44)/(144xx(1000)/(100)=0.1 M` Step 3 To calculate of 0.01 M `KMnO_(4)` solution Applying molarity equatin to balaced redox equation we have `(M_(12)V_(1))/(n_(1))(FeC_(2)O_(4))=(M_(2)V_(2))/(n_(2))(LMnO_(4))` substituting the values of `M_(1)(=0.1),V_(1)(=100)n_(1)=5, M_(2)=(=0.01)` andn=3 , we have `(0.1xx100)/(5)=(0.01xxV_(21))/(3) or V_(2) =(3x0.1xx100)/(5xx0.01)=600 cm^(3)` thus volume of 0.01 M `KMnO_(4)` solution required =600 `cm^(3)` |
|
| 51544. |
19.5 g of benzene on burning in the free supply of oxygen liberated 12.6 kJ of energy at constant pressure. Calculate the enthalpy of combustion of benzene. Write the thermochemical equation. |
|
Answer» Solution :19.5 GRAMS of benzene on burning liberated 126 kl. 78 grams of benzene on burning liberated 502 KJ. The ENTHALPY of combustion of benzene is` -502 kJmol ^(-1).`The thermochemical equation is given as, `C _(6) H _(6(l)) + (15)/(2) O _(2(g)) to 6CO _(2(g)) + 3H_(2(g)) + 3H _(2) O _((l)), Delta H =-502 kJ mol ^(-1)` |
|
| 51545. |
140 mm pressure is developed at equilibrium when PCl_(5) at 100 mm is subjected to dissociation . ThenK_(p) for PCl_(3) + Cl_(2) hArr PCl_(5) is (in atm^(-1)) nearly |
|
Answer» `0.03` |
|
| 51546. |
1,4-pentadiene reacts with excess of HCI in thepresence of benzoyl peroxide to give compound X which upon reaction with excess of Mg in dry ether forms Y. Compound Y on treatment with ethyl acetate followed by dilute acid yields Z. Identify the structures of compound X,Y and Z. |
Answer» SOLUTION :1,4-Pentadiene on addition with excess of HCI in the PRESENCE of benzoyl PEROXIDE, forms 2,4-dichloro pentane (X) because HCI does not SHOW peroxide effect. 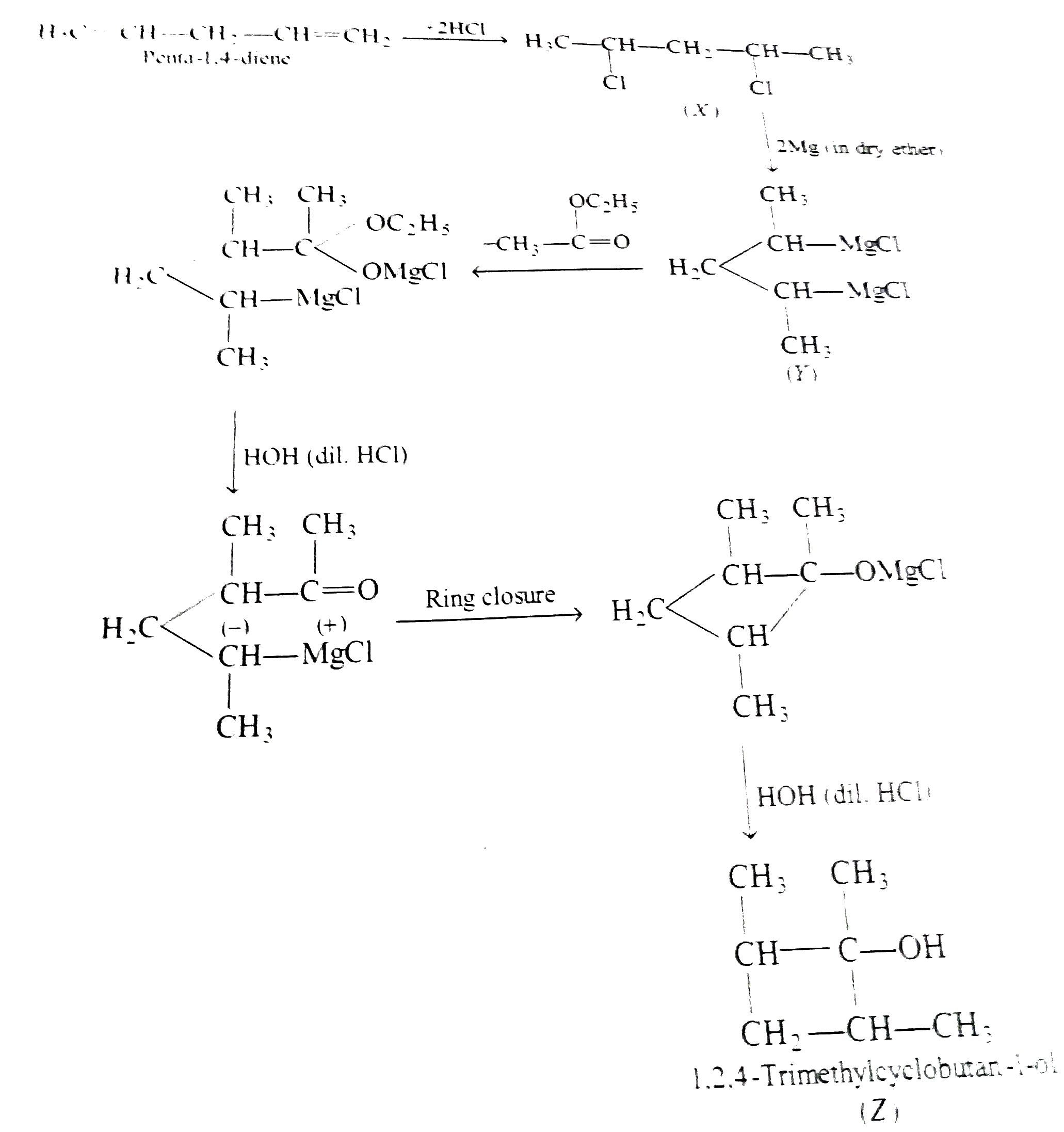
|
|
| 51547. |
14^(@) F is equal to....... |
|
Answer» Solution :`^(@)C = (.^(@)F - 32)/(1.8) = (14-32)/(1.8) = -10^(@) C` `K = .^(@)C + 273 = (-10) + 273 = 263` |
|
| 51548. |
1,4-Dimethylbenzene on heating with anhydrous AlCl_3 and HCl produces |
|
Answer» 1,2-dimethylbenzene 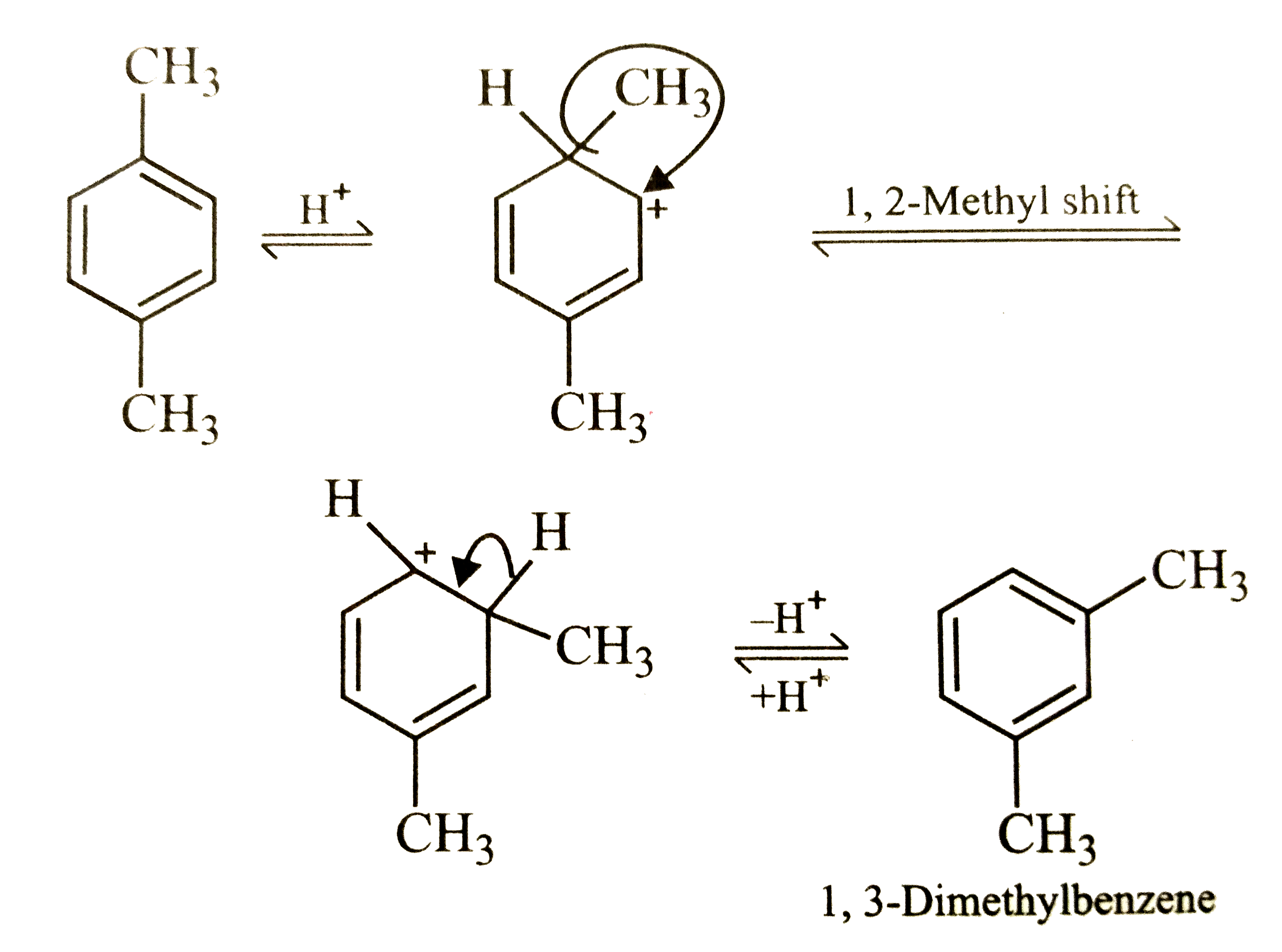
|
|
| 51549. |
13.8g of N_(2)O_(4) was placed in a 1L reaction vessel at 400K and allowed to attain equilibrium N_(2)O_(4)(g)hArr2NO_(2)(g) The total pressure at equilibrium was found to be 9.15 bar . Calcualate K_(c), K_(p) and partial pressure at equilibrium. |
| Answer» SOLUTION :`2.6 MOL L^-1` 85.87 BAR, 8.34 bar | |
| 51550. |
13*8 g " of "N_(2)O_(4) was placed in 1 L reaction vessel in 1 L reaction vessel at 400 K and allowed to attain equilibrium : N_(2)O_(4) (g) hArr 2 NO(g).The total pressure at equilibrium was found to be 9* 15 bar. Calculate K_(c) , K_(p) and partial pressures at equilibrium. |
|
Answer» Solution :`13*8 "g"N_(2)O_(4) = (13*8)/92" mol"=0*15" mol""` `13*8 N_(2)O_(4) = (13*8)/92" mol"=0*15" mol""(Molar mass of "N_(2)O_(4) = 92 g " mol"^(-1))` PV= nRT ` :. P XX 1 L = 0* 15 mol xx 0* 083 " bar"L mol^(-1) K^(-1) xx 400 K or P = 4*98 " bar"` ` {:(,N_(2)O_(4)(g),hArr,2NO_(2)),("INITIAL pressures",4*98 "bar",,0),(" At equilibrium",(4*98-p),,2p):}` ` :. 4*98 - p+ 2 p = 9*15 " bar"or p= 4*17 " bar"` `:. (p_(N_(2)O_(4)))eq = 4* 98 - 4*17 = 0*81 "bar", (p_(NO_(2)))eq = 2 xx 4*17 = 8*34 "bar"` `K_(p) = p_(NO_(2))^(2)//_(P_(N_(2)O_(4)))=(8*34)^(2)//)*81=85*87,` ` K_(p) = K_(c) (RT)^(Delta n) :. 85*87 = K_(c) (0*083 xx400)^(1) or K_(c)= 2*586 = 2*6` |
|