Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 51301. |
25ml of a 0.1M solution of a stable cation of transitional metal Z reacts exactly with 25ml of 0.04M acidified KMnO_4solution. Which of the following is most likely to reperesent the chance in oxidation state of Z correctly |
|
Answer» ` Z^(+ )RARRZ^(2+)` = no . of milli equivalents of `KMnO_(4)` `25 XX 0.1 xx n = 25 xx 0.04 xx 5` `:. `n-factor for .Z. = `2"" :. Z^(2+) rarr Z^(4+)` |
|
| 51302. |
2.5L of a sample of a gas at 27^(@)C and 1 bar pressure is compressed to a volume of 500mL keeping the temperature constnat, the percentage increase in pressure is: |
|
Answer» 1 |
|
| 51303. |
2.5g of a mixture containing CaCO_(3),Ca(HCO_(3))_(2) and NaCl was dissolved in 100mL water and its 10mL portion required 10mL 0.05M H_(2)SO_(4) solution to reach the phenolphthalein end point. Another 10mL portion of the same stock solution required 32.35 mL of the same acid solution to reach the methyl orange end point. Determine mass percentage ratio of CaCO_(3) and Ca(HCO_(3))_(2) in the original mixture. |
|
Answer» |
|
| 51304. |
2.5gms. of a gas is present in 750ml. flask at 32^@C and 770mm, of Hg pressure. Calculate the molecular mass of the gas. |
|
Answer» |
|
| 51305. |
25g of each of the following gases are taken at 27^@C and 600 mm Hg pressure. Which of these will have the least volume ? |
|
Answer» HBr Volume `alpha` Mass/MOLAR mass Volume `alpha` 28/ Molar mass i.e. if molar mass is more, volume isless . HENCE HI has the LEAST volume. |
|
| 51306. |
25g of each of the following gases are taken at 27^(@)C and 600 mm Hg pressure. Which of these will have the least volume ? |
|
Answer» HBr |
|
| 51307. |
2.56g of Sulphur is dissolved in 100g of carbon disulphide. The solution boils at 319.692 K. What is the molecular formula of Sulphur in solution? The boiling point of CS _(2) is 319.450K. Given that K _(b)for CS_(2) = 2.42K kg mol ^(-1) |
|
Answer» Solution :`W_(2)=2.56g` `W_(1)=100 G` ` T = 319.692K` `K _(b) = 2.42 K kg mol ^(-1)` `Delta T_(b) = (319.692-319.450) K = 0.242 K` `M _(2) = (K_(b)xx W_(2) xx 1000)/(Delta T _(b)xx W_(1))= (2.42 xx2.56 xx 1000)/(0.242 xx 100)` `M _(2) = 256 g mol ^(-1)` MOLECULAR mass of SULPHUR in solution `= 256 g mol ^(-1)` Atomic mass of one mole of sulphur atom = 32 No. of atoms in a molecule of sulphur `= (256)/(32) =8` HENCE, molecular formula of sulphure is `S_(8),` |
|
| 51308. |
2.56g of Sulphur is dissolved in 100g of carbon disulphide. The solution boils at 319.692 K. What is the molecular formula of Sulphur in solution. The boiling point of CS_(2) is 319.450K. Given that K_(b) for CS_(2) = 2.42 Kkg mol^(-1) (ii) Show that the sum of mole fraction of a solution is equal to one. |
|
Answer» Solution :`W_(2) = 2.56 g , W_(1) = 100g` `T = 319.692K , K_(b) = 2.42 K kg mol^(-1)` `Delta T_(b) = (319.692- 319.450)K = 0.242K` `M_(2) = (K_(b) xx W_(2) xx 1000)/(Delta T_(b) xx W_(1))= (2.42 xx 2.56 xx 1000)/(0.242 xx 100)` `M_(2) = 256g mol^(-1)` Molecular mass of sulphur in solution = `256g mol^(-1)` Atomic mass of one mole of sulphur atom = 32 No. of atoms in a molecule of sulphur `= (256)/(32) =8` Hence, molecular formula of sulphur is `S_(8)`. (ii) Consider a solution CONTAINING two components A and B WHOSE mole fractions are `x_(A) and x_(B)` respectively. Let the number of moles of two components A and B are `n_(A) and n_(B)` respectively. `x_(A) = (n_(A))/(n_(A) + n_(B)), x_(B) = (n_(B))/(n_(A) + n_(B))` `x_(A) + x_(B) = (n_(A))/(n_(A) + n_(B)) + (n_(B))/(n_(A) + n_(B)) =1` |
|
| 51309. |
25.5 g of H_(2)O_(2) solution on decomposition gave 1.68 L of O_(2) at STP. The percentage strength by weight of the solution is |
|
Answer» 30 |
|
| 51310. |
25.4g of iodine and 14.2g of chlorine are made to react completely to yield a mixture of ICI and ICI_(3). Calcualte the number of moles of Icl and Icl_(3) formed. |
|
Answer» 0.1 MOLE, 0.1 mole |
|
| 51311. |
250mL of 0.2M NaOH and 100mL of 0.5M NaOH solutions were added. What is the molarity of the mixture? |
|
Answer» |
|
| 51312. |
250 mL of nitrogen maintained at 720 mm pressure and 380 mL of oxygen maintained at 650 mm pressure of the misture ? |
|
Answer» Solution :Step 1. To CALCULATE the partial pressure of nitrogen `{:("Given Conditions","Final Conditions"),("VOLUME "V_(1)=250 mL,V_(2)=1000 mL),("Pressure "P_(1)=720 mm,P_(2)=? mm):}` Applying Boyle's Law (since the temperature remains constant), `P_(2)V_(2)=P_(1)V_(1),i.e., "" 1000xxP_(2)=720xx250"or" P_(2)=(720xx250)/(1000)=180 mm` Thus, the partial pressure due to nitrogen `(p_(n_(2)))=180 mm`. Step 2. To calculate the partial pressure of oxygen `{:("Given Conditions","Final Conditions"),(V_(1)=380 mL,V_(2)=1000 mL),(P_(1)=650 mm,P_(2)=? mm):}` Applying Boyle's Law (since the temperature remains constant), `P_(2)V_(2)=P_(1)V_(1),i.e.,1000xxP_(2)=380xx650 "or" P_(2)=(380xx650)/(1000)=247 mm` Thus, the partical pressure due to oxygen `(p_(o)_(2))`=247 mm. Step 3. To calculate the final pressure of the gaseous mixture. If P is the final pressure of the gaseous mixture, then according to Dalton's Law of Partial Pressures, `P=P_(n_(2))+P_(o_(2))=180+247=427 mm`. |
|
| 51313. |
250 mL of 0.2M H_(2)SO_(4) is diluted with one L of water. What is the normality of the dilute acid? |
|
Answer» |
|
| 51314. |
25.0 g of FeSO_(4).7H_(2)O was dissolved in water containing dilute H_(2)SO_(4) and the volume was made up to 1.0 L. 25.0 " mL of " this solution requried 20 " mL of " an (N)/(10) KMnO_(4) solution for complete oxidation the percentage of FeSO_(4)7H_(2)O in the acid solution is |
|
Answer» `78%` 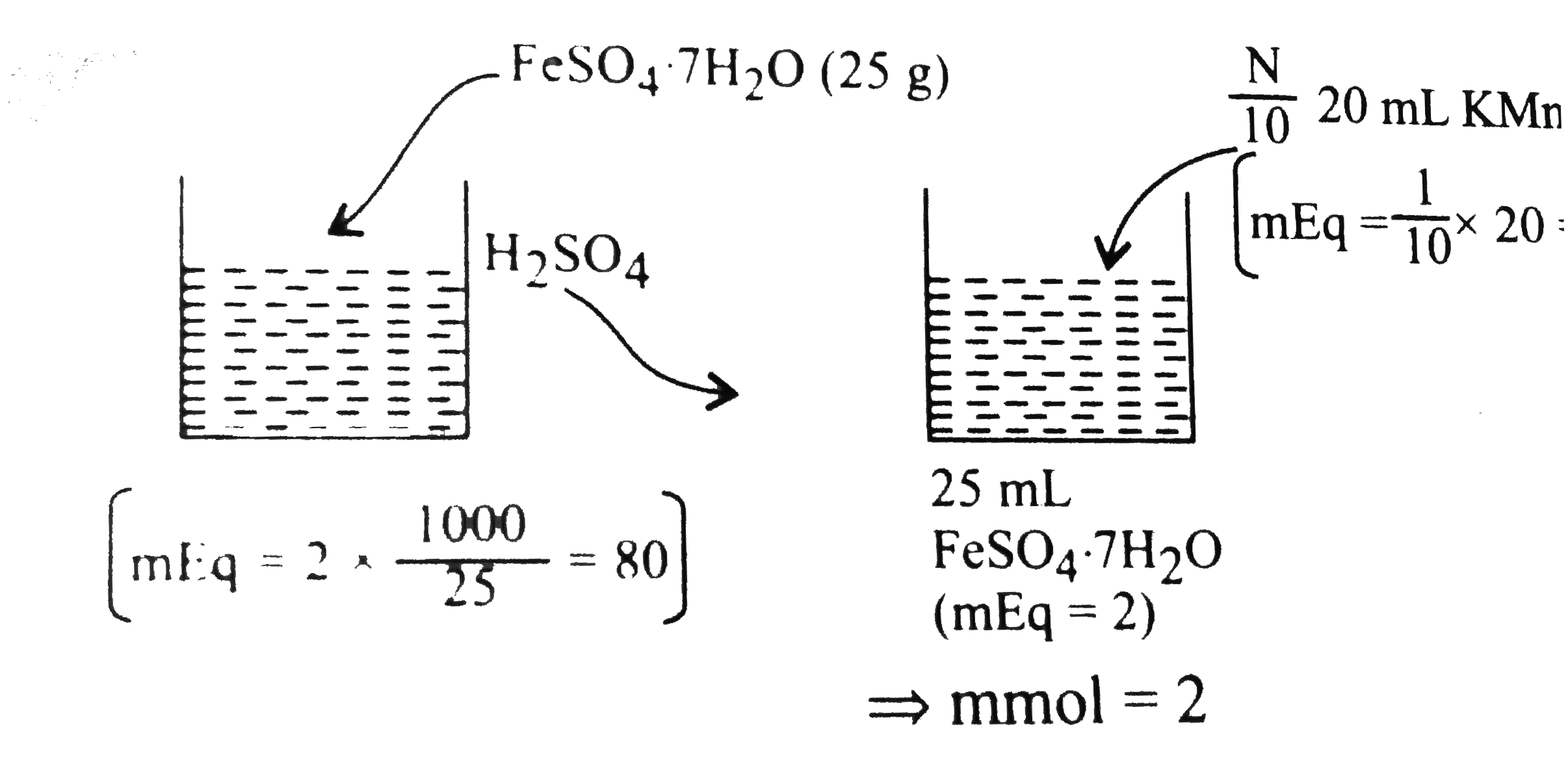 `implies((W_(FeSO_(4).7H_(2)O)xx1000)/((278)/(1))=80` `impliesW_(FeSO_(4).7H_(2)O)=22.24` `implies% of FeSO_(4).6H_(2)O=(22.24)/(25)XX100%=88.96%` |
|
| 51315. |
25.0 cm^(3) of an aqueous solution of H_(2)O was treated with excess of KI soluiton in acidic medium and the liberated iodine required 10.0 cm^(3) of 0.01 M thiosuphte solutoin find out the concentratin of H_(2)O_(2) in gramsper litre ? |
|
Answer» Solution :STEP 1 To write the balanced chemical equation of the redox reaction `2KI + H_(2)SO_(4)+H_(2)O rarr K_(2)SO_(4)+I_(2)+H_(2)O` `2Na_(2)S_(2)O_(3)+I_(2)rarrNa_(2)S_(4)O_(6)+2Nal` `2KI+H_(2)SO_(4)+2 Na_(2)S_(2)O_(3)+H_(2)O_(2)rarrK_(2)SO_(4)+Na_(2)S_(4)O_(6)+2 Nal + 2 H_(2)O` From the above equation Step 2 To FIND out hte concentration fo `HH_(2)O_(2)` Let the molarity f `H_(2)O_(2)` solution =`M_(1)` Applying molarity equaition `(M_(1)xx25)/(1)=(10xx0.1)/(2)M_(1)=(10xx0.1)/(2xx25)=0.02M` Mol wt of `H_(2)O_(2)=2xx1+2xx16=34` `therefore` concentration of `H_(2)O_(2)=0.02xx34 =0.68 g L^(-1)` |
|
| 51316. |
25.0 cm^(3) of a solution containing 15.0 g of a partially oxidisied sample of green vitrion (FeSO_(4).7H_(2)O) per litre required 20.0 cm^(3) mL of 0.01 M potassium dichromate solution for oxidiation in aidic medium find out the percentage purity of the given sample of green vitriol |
|
Answer» Solution :Step 1 To write balaced EQUATION for the redox reaction `K_(2)Cr_(2)+4 H_(2)SO_(4) rarr K_(2)SO_(4)+Cr_(2)(sO_(4))_(3)+4H_(2)O+3O` `2FeSO_(4)+H_(2)SO_(4)+OrarrFe_(2)(SO_(4))+H_(2)O[xx3` `K_(2)Cr_(2)O_(7)+6FeSO_(4)+7H_(2)SO_(4)rarr K_(2)SO_(4)+Cr_(2)SO_(4)^(3)+3Fe_(2)SO_(4)^(3)+7H_(2)O` From the above equation 1 MOLE of `K_(2)Cr_(2)O_(7)=6` MOLES of `FeSO_(4)` Step 2 To find percentage purity of green vitroit Let `M_(1)` be the molarity of the oxidised sample of green vitril applying molarity eqaution we have `(M_(1)xx25)/(6)(FeSO_(4))=(20xx0.1)/(1)(K_(2)Cr_(2)O_(7))` `M_(1)=(20xx0.01xx6)/(25)=0.048 M` Mol wt of `FeSO_(4)7H_(2)O=56+32+4xx16+7xx18=278` Wt of pure `FeSO_(4), 7H_(2)O=278xx0.048=13.344 g L^(-1)` % purity of green vitriol `=(13.444)/(15)xx100=88.96` |
|
| 51317. |
25 mL of N/10 NaOHsolution exactly neutralise 20 mL of an acid solution. What is the normality of the acid solution ? |
|
Answer» Solution :According to the normality relation, `underset("NaOH")(N_(1)V_(1)) = underset("acid")(N_(2)V_(2))` In the present CASE, `N_(1) = 1/10, V_(1) = 25 ML, N_(2) = ?,V_(2) = 20 mL` SUBSTITUTING the values, we have `1/10 xx 25 = N_(2) xx 20` `therefore N_(2) = 25/(10 xx 20)= 0.125` Hence, the normality of the acid solution is 0.125 N |
|
| 51318. |
25 ml of hydrogen peroxide solution were added to excess of acidified potassiutm iodide solution. The iodine so liberated required 20mL of 0.1 sodium this sulphate solution. Calculate the strenth in terms of normality, percentage and volume. |
|
Answer» Solution :Let the molarity of `H_(2)O_(2)=N_(x)` 20mL of 0.1 N `Na_(2)SO_(3)-=20mL of 0.1 I_(2)=25mL.N_(x).H_(2)O_(2)` Normality of `H_(2)O_(2)(N_(x))=(20xx0.1)/(25)=0.08N` STRENGTH of `H_(2)O_(2)`=Normality`xx`Equivalent mass=`0.08xx17=1.36gL^(-1)` Percentage of `H_(2)O_(2)=(1.36)/(1000)xx100=0.136` This means that 0.136g of `H_(2)O_(2)` are PRESENT in 100mL solution or 0.00136g of `H_(2)O_(2)` are present in 1ML of the solution Now, 68g of `H_(2)O_(2)` give oxygen at N.T.P. =22400mL 0.00136g of `H_(2)O_(2)` given oxygen at N.T.P.`=(22400)/(68)xx0.00136=0.448mL` Thus. 1mL of `H_(2)O_(2)` givwes 0.048mL of oxgyen at N.T.P. |
|
| 51319. |
25 mLof household bleach solution was mixed with 30 mL of 0.50 M KI and 10 mL of 4 Nacetic acid in the titration of the liberated iodine 48 mL of 0.25 N Na_(2)S_(2)O_(3) was used to reach the end point the molarity of the household bleach solution is |
|
Answer» 0.48 M `CA(OCI)_(2)+4H^(+)+2e^(-)rarrCa^(2)+2H_(2)O+CI_(2)` `2KI+CI_(2)rarr2KCI+I_(2)` `2Na_(2)S_(2)O_(3)+I_(2)rarrNa_(2)S_(4)O_(6)+2` Nal `Ca(OCI_(2))+4H^(+)+2e^(-)+2Na_(2)S_(2)O_(3)rarrCa^(2+)+2H_(2)O+2KCI+Na_(2)S_(4)O_(6)+2NaI` Thus the normaility of`Ca(OCI)_(2)` solution =0.48 N Now since EQ (i) is `2e^(-)` change `therefore`Eq wt of `Ca(OcI)_(2)="Mol wt"//2` `=0.48//2` thus the molarity of the household BLEACH =0.24 M |
|
| 51320. |
25 ml of H_(2)O_(2) solution were added to excess of acidified KI solution. The iodine so liberated required 20 ml of 0.1 N Na_(2)S_(2)O_(3) solution. Calculate strength in terms of normality and percentage. |
|
Answer» 0.04 N, `0.136%` `2(S^(+2))_(2) rarr (S^(+5//2))_(4)+2e^(-), I_(2)+2e^(-) rarr 2I^(-)` Meq. Of `H_(2)O_(2)=` Meq. of `I_(2)=` Meq. of `Na_(2)S_(2)O_(3)` `w/(34//2) times 1000=20 times 0.1` `therefore W_(H_(2)O_(2))=0.034 gr//25ml` `therefore N_(H_(2)O_(2))=0.034/(34//2) times 1000/25=0.08` Vol. STRENGTH `=5.6 times 0.08=0.448` % strength `=17/56 times 5.6 times 0.08=0.136%` |
|
| 51321. |
25 ml of H_(2)O_(2) solution was added to excess of acidified Kl solution. The iodine so liberated required 20 ml. of 1 N sodium thiosulphate solution. What is volume strength ofH_(2)O_(2) |
|
Answer» 0.224 ` = 20 mL 0.1 NH_2O_2` Normality of ADDED `H_2O_2 = (20 xx 0.1)/25 = 0.08` vol. strength`= 5.6 xx 0.08 = 0.448` |
|
| 51322. |
25 mL of a solution of barium hydroxide on titration with a 0.1 molar solution of hydrochloric acid gave a titre value of 35 mL. The molarity of barium hydroxide solution was : |
|
Answer» 0.28 No. of moles of `HCl=(("0.1 mol"^(-1)))/(("1L"))xx("0.035L")` `=3.5xx10^(-3)` mol No. of moles of `Ba(OH)_(2)=(3.5xx10^(-3))/(2)` `=1.75xx10^(-3)` mol MOLARITY of `Ba(OH)_(2)` solution `= ("No. of moles of Ba"("OH")_(2))/("Volume of solution in litres")` `=((1.75xx10^(-3)"mol"))/(("0.025 L"))=0.07"mol L"^(-1)` `=0.07M`. |
|
| 51323. |
25 mL of a mixture of NaOH+Na_(2)CO_(3), when titrated with (N)/(10)HCl using phenolphthalein indicator required 25 mL HCl to deccolourise phenolphthalein. At this stage methyl orange was added and addition of acid was continued. The second end point was reached after further addition of 5 mL of the acid. Calculate the amount of Na_(2)CO_(3) and NaOH in one litre of the solution. |
|
Answer» SOLUTION :Between first and second end points, `NaHCO_(3)+HCl to NaCl+H_(2)CO_(3)` `5 mL (N)/(10)HCl-=(1)/(2)Na_(2)CO_(3)` present in 25 mL of a mixture or `10 mL (N)/(10)HCl-=Na_(2)CO_(3)` present in 25 mL of a mixture `-=10 mL (N)/(10)Na_(2)CO_(3)=0.053 G Na_(2)CO_(3)` Amount of `Na_(2)CO_(3)` in one litre of mixture `=(0.053)/(25)xx1000=2.12 g` `(25-5)mL(N)/(10)HCl-=NaOH` present in 25 mL of mixture `-=25 mL (N)/(10)NaOH` `-=0.08 gNaOH` Amount of NaOH in one litre of mixture`=(0.08)/(25)xx1000=3.2 g` |
|
| 51324. |
25 mL of a mixed solution of sodium carbonate and sodium bicarbonate required 10 mL of N//20 HCl when titrated in the presence of phenolphthalein but 25 mL of the same when titrated separately in presence of methyl orange required 25 mL of N//10 HCl. Calculate the amount of anhydrous sodium carbonate and bicarbonate in grams per litre of the solution. |
|
Answer» |
|
| 51325. |
25 mL of a gaseous hydrocarbon is mixed with 150mL of oxygen in an eudiometer. After sparking and cooling back the mixture is passed through alkaline pyrogallol. The volume loss surffered in pyrogallol is 25mL. Calculate the molecular weight of hydrocarbon. |
|
Answer» Solution :VOLUME of oxygen taken = 150mL Volume of unreacted oxygen, absorbed in pyrogallol = 25ML Ratio of volumes of HYDROCARBON and oxygen = 25mL : 125mL = 1:5 One volume of hydrocarbon consumes five volumes of oxygen for combustion. The gaseous hydrocarbon is `C_(3)H_(8)` Molecular weight of the gaseous hydrocarbon = 44 |
|
| 51326. |
25 mL of a mixture of NaOH and Na_(2)CO_(3) when itrated with N//10 HCl using phenolphthalein indicator required 25 mL HCl. The same volume of mixture when titrated with N//10 HCl using methyl orange indicator required 30 mL of HCl. Calculate the amount of Na_(2)CO_(3) and NaOH in one litre of this mixture. |
|
Answer» Solution :When phenolphthalein is the indicator, whole of NaOH has been neutralised and carbonate converted into bicarbonate, i.e., `NaOH+HCL to NaCl+H_(2)O` `Na_(2)CO_(3)+HCl to NaHCO_(3)+NaCl` So, `25 mL (N)/(10)HCl-=NaOH+1//2Na_(2)CO_(3)` present in 25 mL of mixture In ANOTHER titration when methyl orange is the indicator, whole of NaOH has been neutralised and carbonate converted into carbonic acid, i.e., `Na_(2)CO_(3)+2HCl to 2NaCl +H_(2)CO_(3)` `30 mL(N)/(10) HCl -=NaOH+Na_(2)CO_(3)` present in 25 mL of mixture Hence, `(30-25)mL (N)/(10)HCl-=(1)/(2)Na_(2)CO_(3)` present in 25 mL of mixture Hence, `10 mL (N)/(10)HCl-=Na_(2)CO_(3)` present in 25 mL of mixture `-=10 mL(N)/(10)Na_(2)CO_(3)` solution Amount of `Na_(2)CO_(3)=(53xx10)/(10xx1000)=0.053 g` This amount of `Na_(2)CO_(3)` is present in 25 mL of mixture. The amount present in one litre of mixture `=(0.053)/(25)xx1000=2.12g` `(30-10)mL (N)/(10)HCl-=NaOH` present in 25 mL of mixture `-=20 mL(N)/(10)NaOH` Amount of NaOH in 25 mL of mixture`=(40xx20)/(10xx1000)=0.08 g` The amount present in one litre of mixture`=(0.08)/(25)xx1000=3.20 g` |
|
| 51327. |
2.5 mL of 2//5 M weak monoacidic base (K_(b)=1xx10^(-12) at 25^(@)C) is titrated with 2//15 M HCI in water at 25^(@)C. The concentration of H^(+) at equivalence point is (K_(w)=1xx10^(-14) at 25^(@)C) |
|
Answer» `2.7xx10^(-2)M` At the equivalence point, all `NH_(4)OH` gets converted into `NH_(4)Cl` (salt) which undergoes cationic hydrolysis as follows: `{:(,NH_(4)^(+)(aq.)+H_(2)O(l)hArrNH_(4)OH(aq.)+H^(+)(aq.)),("Initial (M)","C00"),("Change (M)", "-Ch+Ch+Ch"),("Equilibrium (M)",bar("C(1-h)ChCh")):}` Thus, the concentration of `H^(+)` ions is due to the hydrolysis of the resulting salt `(NH_(4)Cl)`. To calculate is value, we need the concentration of salt (C ) and the degree of hydrolysis of salt (h). `"Concentration of salt" = ("Millimoles of base")/("TOTAL volume")` Millimoles of base `=M.V_(mL)` `=((2)/(5))(2.5)` Total volume `=V_("acid")+V_("base")` `V_("acid")=(M_("base")V_("base"))/(M_("acid"))` `=((2//5)(2.5))/((2//15))=7.5 mL` `:. C_("salt")=((2//5)(2.5))/((7.5+2.5))=(1)/(10)=0.1` For cationic hydrolysis, `K_(h)=(Ch^(2))/(1-h)=(K_(w))/(K_(b))=(10^(-14))/(10^(-12))=10^(-2)` We have `C=0.1`, `K_(w)=1xx10^(-14)`, `K_(b)=1xx10^(-12)` This gives `h=0.27` (since `K_(h)` is not neglected relative to 1) `C_(H^(+))=Ch` `=(0.1)(0.27)` `=2.7xx10^(-2)M` If we neglact h relative 1, the answer will be `3.2xx10^(-2)`M. |
|
| 51328. |
2.5mL of (2)/(5) M weak monoacidic base (K_(b)=1xx10^(-12) "at" 25^(@)C) is titrated with (2)/(15) M HCl in water at 25^(@) C. The concentration of H^(+) at equivalence point is (K_(w) = 1 xx 10^(-14)"at"25^(@)C) |
|
Answer» `3.7xx10^(-13)M` `2.5xx(2)/(5)=(2)/(15)=(2)/(15)xxV_(2) "or" V_(2)=(15)/(2) ML = 7.5 mL ` `BOH+HClrarrBCl+H_(2)O` 2.5 mL of 2 M base contain base `=2.5xx(2)/(5) = 1` mmol `:. ` Salt BCl formed = 1 mmol Volume of solution = 2.5 mL + 7.5 mL = 10 mL `:.` Conc. of salt [BCl] in the solution `=(1)/(10)M=0.1M` For salt of weak base and STRONG acid `[H^(+)]=sqrt((K_(w)C)/(K_(b)))=sqrt((10^(-14)xx0.1)/(10^(-12)))=3.2xx10^(-2)M` |
|
| 51329. |
25 mL of 0.1 M solution of metallic salt (A) oxidised 25 mL of 0.1 M sodium sulphite to sodium sulphate. If oxidation number of the metal in the salt (A) is 3, then new oxidation number of the metal is __________ |
|
Answer» |
|
| 51330. |
25 mL N K_(2)Cr_(2)O_(7) acidified solution will liberate .. Iodine from KI solution. |
|
Answer» 0.3175 g |
|
| 51331. |
25 mL H_(2)O_(2) were added to excess of acidified solution of KI. The iodine so liberated required 20 mL of 0.1 N sodium thisulphate for titration. Calculate the strength in terms of normality percentage and volume. |
|
Answer» |
|
| 51332. |
25 mL3MHNO_(3) and 75 mL 4 M HNO_(3) solutions are mixed. Then the molarity of the resulting solution is .......... M. |
|
Answer» 3.5 M `M=(M_(1)V_(1)+M_(2)V_(2))/(V_(1)+V_(2))=(3xx25+4xx75)/(25+75)` `= (375)/(100)= 3.75` |
|
| 51333. |
25 mL 0.1 N H_(2)SO_(4) neutralized with 20 mL xN Na_(2)CO_(3).What will be the g/liter of Na_(2)CO_(3) ? |
|
Answer» Solution :`N_(1)V_(1) =N_(2)V_(2)` `(0.1)(25) =(X)(20)` `:.x=(0.1xx25)/(20)` `:.N_(2)=0.125` `:.0.125 N Na_(2)CO_(3) = (0.125)/(2)` `= 0.0625 M Na_(2)CO_(3)` solution `= 0.0625xx10^(6) g//L` `=6.625 g` |
|
| 51334. |
2.5 litres of 1M NaOH solution is mixed with 3.0 litres of 0.5 M NaOH solution. The molarity of resulting solution is : |
|
Answer» 0.80 M or `M_(3)=(M_(1)V_(1)+M_(3)V_(3))/(V_(3))` `=(1xx2.5+0.5xx3.0)/(5.5)=0.73M` |
|
| 51335. |
2.5 L of 1 M NaOH solution is mixed with another 3 L solution of 0.5 M NaOH solution Then the molarity of the resulting solution is.... |
|
Answer» 0.560 M `=(M_(1)V_(1)+M_(2)V_(2))/(V_(1)+V_(2))=(1xx2.5+0.5xx3)/(5.5)` `=0.727~~0.73M` |
|
| 51336. |
2.5 grams of a divalent metal carbonate on treating with excess dilute mineral acid liberated a gas,which was measured at 1 atm and 273K as 5.6L. Calculate the atomic weight of the metal present in the given salt. |
|
Answer» |
|
| 51337. |
2.5 grams of a sample of chalk is strongly heated . If 0.88 grams of carbondioxide is produced, What is the percentage purity of CaCO_(3) in the sample? |
|
Answer» |
|
| 51338. |
25 g of an unknown hydrocarbon upon burning produces 88 g of CO_2and 9 g of H_2O. This unknown hydrocarbon contains |
|
Answer» 18 g of carbon and 7 g of HYDROGEN |
|
| 51339. |
25 g of a sample of ferrous sulphate was dissolved in water containing dilute H_(2)SO_(4) and the volume made up to one litre. 25 mL of this solution required 20 mL of N//10 KMnO_(4) solution for complete oxidation. Calculate the percentage of FeSO_(4). 7H_(2)O in the sample. |
|
Answer» |
|
| 51340. |
2.480g of KcIO_(3) are dissolved in conc. HCl and the solution was boiled. Chorine gas evolved in the reactionwas then passed through a solution of Ki and libeerated iondine was titrated with 100mL of hypo 12.3mL of same hypo solution required 26.6 mL of 0.5N idone for complete neutralization. Calculate % purity of KCIO_(3) sample. |
|
Answer» |
|
| 51341. |
2.48 g of Na_(2)S_(2)O_(3)xH_(2)O was dissolved per litre of the solution 20mL of tis solution required 10 ml 0.01 m iodin e solution find out the value of x |
|
Answer» Let the molarity of `Na_(2)S_(2)O_(3)xH_(2)O "solution" =M_(1)` Applying molarity equation to the above redox reaction we have `(M_(1)xx20)/(2)(Na_(2)S_(2)O_(3))=(10xx.01)/(1)(I_(2))` `therefore M_(1)=0.01 M` But the actual amount dissolved =2.48g Equating these VALUES we have `(158+18x)xx0.01=2.48 or x=5` |
|
| 51342. |
2.48 g of hydrated sodium thiosulphate (Na_2S_2O_3 .xH_2O) was dissolved per litre of the solution. 25 mL of this solution required 12.5 mL of M /100 iodine solution . Determine the value of x. |
|
Answer» SOLUTION :The balanced chemical equation is : `2Na_2S_2O_3+I_2 rarr Na_2S_4O_6+2NaI` Let the MORALITY of `Na_2S_2O_3.xH_2O` be `M_1` Applying morality equation `((M_1V_1)/n_1)_(Na_2S_2O_3)=((M_2V_2)/n_2)_(I_2)` `(M_1xx25)/2-=1/100xx(12.5)/1` `M_1=(12.5xx2)/(100xx25)=0.01M` Molecular WT. of `Na_2S_2O_3.xH_2O` `=2xx23+2xx32+3xx16+xxx18` Amount of `Na_2S_2O_3.xH_2O=0.01xx(158+18x)g` But actural amount = 2.48 g `:.0.01(158+18x)=2.48` `(158+18x)=(2.48)/(0.01)=248` `18x=248-158=90` `:. x = 90 / 18 =5`. |
|
| 51343. |
2.46 g of sodium hydroxide (molar mass = 40) are dissolved in water and the solution is made to 100 cm^3 in a volumetric flask. Calculate the molarity of the solution. |
|
Answer» |
|
| 51344. |
240cc of SO_2 gas diffused through a porous membrane in 20 minutes. Under similar conditions, 720 cc of another gas diffused in 30 minutes. Find the molecular mass of the gas. |
|
Answer» |
|
| 51345. |
240 mL of a dry gas measured at 27°C and 750 mm pressure weighed 0.64 g. What is the molecular mass of the gas ? |
|
Answer» |
|
| 51346. |
24 ml of water gas containing only hydrogen and carbon monoxide in equal proportions by volume are exploded with 80 ml of air in which 20% by volumeis O_2 , if all gasesous are measured at room temperature and pressure , calculate the composition by volume of the unreacted resulting gaseous mixture . |
|
Answer» `O_(2) to 12 mlN_(2) to 64 ml CO_(2) to4 ml` (II) `underset(" 2 volume") underset(12) (2 CO) + underset(" 1 volume") underset(6) (O_(2)) to underset(2 " volume") (2 CO_(2)) (12 ml)` Total OXYGEN USED 6 +6= 12 ml Total oxygen PRESENT in 80 ml of air = `80 xx 20//100 = 16 ` ml Un used = 4 ml `N_(2) = 64` ml `CO_(2) = 12` ml |
|
| 51347. |
2.4 mole ofH_2 sample was taken . In one experiment60% of the sample was exposed to conitnuous radiations of frequency4.47 xx 10 Hz, of which all he electrons are removed from the atom . In another experiment remaining sample was irradiated with light of wavelength600Å , when all the electrons are removed from the surface , Calculate the ratio of maximum velocity of the ejected electron in the two cases . Also report the velocity of ejected electron in each case . Assume that ejected electrons does not interact wih any photon . (Ionization potential ofH= 13. 6 eV). |
| Answer» SOLUTION :` 0. 83 or 1.22 ,` | |
| 51348. |
24 gm of optically pure tartaric acid is dissolved in water to make 240 ml solution. It is kept in 20 cm polarimeter tube and plan polarised light is passed through it to produce rotation of -2.4^(@). Answer the following questions : If origihnal solution is diluted by 2 times and length of polarimeter is increasexd four times of previous length. What will be the specific rotation? |
|
Answer» -4.8 |
|
| 51349. |
24 gm of optically pure tartaric acid is dissolved in water to make 240 ml solution. It is kept in 20 cm polarimeter tube and plan polarised light is passed through it to produce rotation of -2.4^(@). Answer the following questions : If mixture of d and l tartaric acid has the specific rotation - 4.0^(@), calculate the % of potical purity of this mixture? |
|
Answer» 0.5 |
|
| 51350. |
24 gm of optically pure tartaric acid is dissolved in water to make 240 ml solution. It is kept in 20 cm polarimeter tube and plan polarised light is passed through it to produce rotation of -2.4^(@). Answer the following questions : Calculate the % of d tartaric acid in a mixture of d and l tartaric acid with has the observed specific rotation + 6.0^(@). |
|
Answer» 0.25 |
|