Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 51351. |
24 g of magnesium in the vapour state absorb 1200 kJ of energy. If IE_1 and IE_2 of magnesium are 750 and 1450 kJ mol^(-1) respectively, the final composition of mixture is |
|
Answer» 0.69 MOL `MG^(+)` and 0.31 mol `Mg^(2+)` `:.` Amount of Mg=1 mol THUS, 750 kJ of energy is used up to convert 1 mol of Mg to `Mg^(+)` Energy used to convert `Mg^(+)` to `Mg^(2+)` `=1200-750 =450 kJ` `:.` Amount of magnesium converted to `Mg^(2+)` `=(450 kJ)/(1450) =0.31 mol` `:. ` Amount of magnesium converted to `Mg^(+)` =1-0.31 =0.69 mol |
|
| 51352. |
23g of Na will react with ethanol to give |
|
Answer» ONE MOLE of oxygen 23gof Na gives `H_(2) = 0.5 MOL`. |
|
| 51353. |
23g of sodium will react with methyl alcohol to give |
|
Answer» one mole of oxygen `23g` of `Na -= 1 mol Na -= (1)/(2) mol H_(2)` `= (1)/(2) xx 22.4 = 11.2 L` of `H_(2)` at N.T.P. |
|
| 51354. |
2,3-dimethylhexane contains......tertiary......secondary and...primary carbons respectively. |
|
Answer» 2,2,4 |
|
| 51355. |
2,3-Dimethyl-2-butene can be prepared by heating which of the following compounds with a strong acid ? |
|
Answer» `(CH_(3))_(2)C=CH-CH_(2)-CH_(3)` 
|
|
| 51356. |
2,3-Dimethyl-2-butene can be prepared by heated which of the following compounds with a strong acid ? |
|
Answer» `(CH_3)_3C-CH=CH_2` 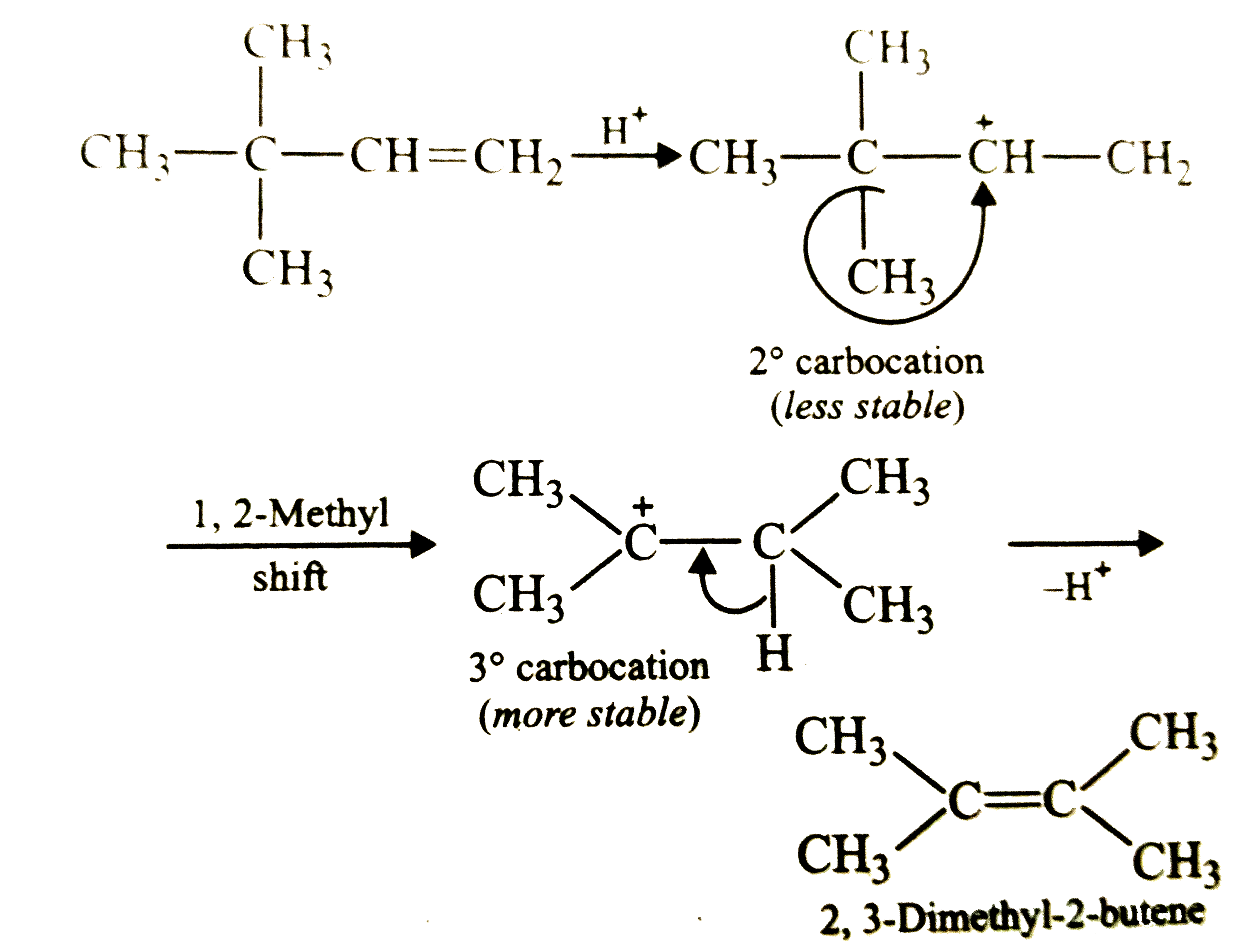
|
|
| 51357. |
2,3-Butanediol has the 2R, 3R configuration Identify the correct statement among the following. |
|
Answer» 2R, 3S - is its ENANTIOMERS |
|
| 51358. |
.^(227)Ac has a half-line of 22 year with respect to radiioactive decay. The decay follows two parallel paths, one leading to .^(227)Th and the other leading to .^(223)Fr. The percentage yiedls of these two daughter nuclides are 2% and 98.0% respectovely. What is the rate constant in "year"^(-1), for each of the separate paths? |
|
Answer» Solution :We know, `lambda_(av) = (0.693)/(22) = 3.15xx10^(-2) "year"^(-1)` For the decay involving TWO PARALLEL paths, 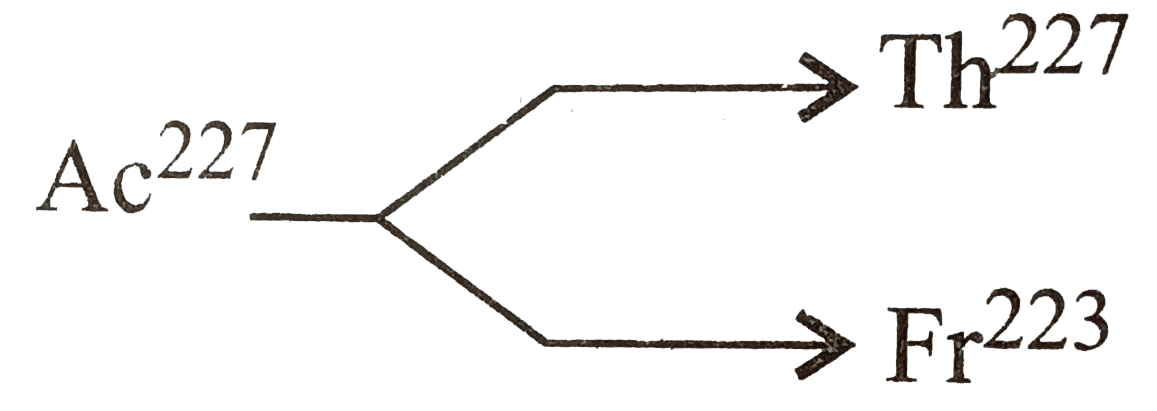 We have `lambda_(AC) = lambda_("Th path") + lambda_("Fr path")` `:. Lambda_(Ac) xx "Fraction of Th" = lambda_("Th path")` ....(1) `lambda_(AC) xx "Fraction of Fr" = lambda_("Fr path")` .....(2) or `lambda_(AC) xx (1 - "Fraction of Th") = lambda_("Fr path")` .....(3) Thus by eqs. (1) and (3) we get `lambda_(AC) =lambda_("Th path") + lambda_("Fr path")` Thus by eqs. (1) and (3) we get `lambda_(AC) = lambda_("Th path")+ lambda_("Fr path")` Thus, Fractionlal yield of `Th = (lambda_("Th path"))/(lambda_("Ac path"))` or `lambda_("Th path")=3.15xx10^(-2)xx(2)/(100)` `=6.30xx10^(-4)yr^(-1)` Also Fractional yeild of Fr`=(lambda_("Fr path"))/(lambda_(Ac path))` `:. lambda_(Fr) = 3.15xx10^(-2) xx (98)/(100) =3.087xx10^(-2) yr^(-1)` |
|
| 51359. |
22.71 Lit means ………. meter ? |
|
Answer» Solution :`22.71 L = 22.71 xx10^(-3)m^(3)` 1 LIT `= 1 (DM)^(3)` `1 dm = 0.1 m = 1xx10^(-1)m` `1 (dm)^(3)=(0.1 m)^(3)=1xx10^(-3)m^(3)` `1L=1(dm)^(3)=1xx10^(-3)m^(3)` `therefore 22.71 L =(22.71 L xx1xx10^(-3)m^(3))/(12)=22.71xx10^(-3)m^(3)` gas Molar VOLUME `= 22.71 L = 22.71xx10^(-3)m^(3)` |
|
| 51360. |
2.26 g of impure ammonium chloride were boiled with 100 mL of NaOH solution till no more ammonia was given off. The excess of NaOH solution left over required 30 mL 2N H_(2)SO_(4) for neutralisation. Calculate the percentage purity of the salt. (H=1, N=14, O=16, Na=23, S=32, Cl=35.5) |
|
Answer» |
|
| 51361. |
22.44 kJ energy is required to convert 8 gm of gaseous atom of metal M to M_((g))^+ if I.E._1 of metal M = 374 kJ/mole. Select correct for above metal M |
|
Answer» 0.6 MOLE gaseous ion (`M^+`) are formed |
|
| 51362. |
22.4 litres of water vapour at NTP, when condensed to water, occupies an approximate volume of: |
|
Answer» 18 litres |
|
| 51364. |
2.20 g of an ammonium salt were boiled with 75 mL of NaOH till the emission of ammonia gas ceased. The excess of unused NaOH solution required 70 mL of N//2 sulphuric acid for neutralisation. Calculate the percentage of ammonia in the salt. |
|
Answer» |
|
| 51365. |
2.2 moles of phosphorus penta chloride were taken in a closed vessel and dissociated into phosphorus trichloride and chlorine . At equilibrium , the total number of moles of the reactants and the products was 2.53 . The degree of dissociation is , |
|
Answer» 0.33 |
|
| 51366. |
Both 22 grams of CO_(2) and 8 grams of methane contain the following carbon atoms |
| Answer» Answer :C | |
| 51367. |
22 g of CO_(2) contains ……….. molecules of CO_(2). |
|
Answer» `:.`22 g of `CO_(2)` will contain = `(6.023xx10^(23))/44xx22 = 3.0115 xx 10^(23)` |
|
| 51368. |
22 g of a gas occupies 11.2 litres of volume at STP. The gas is |
|
Answer» CH4 11.2 litres is occupied by 22 g of a gas.`:.` Molar volume 22.4 LITRE will be occupied by`22/11.2xx22.4 = 44` g `:.` The gas is C`O_(2)` . |
|
| 51369. |
2.18g of an organic compound containing sulphur produces 1.02 g of BaSO_(4). The percentage of sulphur in the compound is |
|
Answer» 0.0726 |
|
| 51370. |
2.16 grams of Cu on reaction with HNO_(3) followed by ignition of the nitrate gave 2.7 gm of copper oxide. In another experiment 1.15 gm of copper oxide upon reduction with hydrogen gave 0.92 gm of copper. This data illustrate the law of |
|
Answer» MULTIPLE Proportions |
|
| 51371. |
21 Mol of FeSO_(2) (atomic weight of Fe is 55.84 g mol^(-1)) is oxidized to Fe_(2)(SO_(4))^(3) calculate theequivalent weight of ferrous ion |
|
Answer» 55.84 |
|
| 51372. |
2.1 g of metal carbonate on thermal decomposition gave 1 g of metal oxide as residue. Determine theequivalent weight of metal. |
|
Answer» Solution :Let the EQUIVALENT weight of metal=x Equivalent weight of metal oxide =x+8 Equivalent weight of metal CARBONATE =x+30 When weight RATIO is related to equivalent weight ratio for two substances `(W_(i))/(W_(2))=(E_(1))/(E_(2)), (2.1)/(1)=(x+30)/(x+8)` SOLVING , x = 12 Equivalent weight of metal = 12 |
|
| 51373. |
20ml of hydrogen peroxide solution is added to excess of acidified KI solution. The iodine so liberated requires 20ml of 0.1M hypo solution. Calculate the molarity and percentage strength of H_2O_2solution. |
| Answer» SOLUTION :0.05M, 0.17 (w/v)% | |
| 51374. |
20mL of each 0.1M NH,OH and IM NH CI aqueous solutions form a buffer of pH 8.3. Calculate the equilibrium constant for NH_4 OH hArr NH_(4)^(+)+ OH^(-). |
| Answer» SOLUTION :`2 XX 10^(-5)` | |
| 51375. |
20mL fo 0.2M MbnSO_(4) are completely oxidiz3d by 16 mL of KMnO_(4) of unknown normaliity each fromingMn^(4+) oxidation state. Find out the normality and molarity of KMnO_(4) solution. |
|
Answer» |
|
| 51376. |
20g of sample containing Ba(OH)_(2) is dissolved in 10 ml of 0.5 MHCl solution. The excess of HCl was then titrated against 0.2 M NaOH. The volume of NaOH used in the titration was 10 ml. Calculate the percentage of Ba(OH)_(2) in the sample. (Mol. wt. of Ba(OH_(2))=171) |
|
Answer» Solution :Calculation of volume of HCl used in titration between NAOH and HCl. `V_(NaOH) =10 ML, M_(NaOH) = 0.2 M, M_(HC) = 0.5 M, V_(HCl)`=? `M_(NaOH) xx V_(NaOH) = M_(HCl) xx V_(HCl), V_(HCl) = (10 xx 0.2)/0.5 = 4 ml` This is the volume of HCl left unused when excess of HCl is added to `Ba(OH)_(2)` solution. Total volume of HCl added = 10 ml Volume of HCl used to react with `Ba(OH)_(2) = 10-4 = 6` ml `2HCl + Ba(OH)_(2) to BaCl_(2) + 2H_(2)O` M = No. of moles x `1000/("volume of solution") rArr 0.5 = "moles" xx 1000/6`. `(0.5 xx 6)/1000` = moles of HCl Moles of HCl used = 0.003 moles. Observing the molar ratio of HCl and `Ba(OH)_(2)`. Moles of `Ba(OH)_(2)` REACTED `=1/2` x moles of HCl reacted `=1/2 xx 0.003 = 0.0015` moles. Weight of `Ba(OH)_(2)` reacted = no. of moles x mol. WT. =` 0.0015 xx 171 = 0.2565` g Percentage of `Ba(OH)_(2)` in the sample `=(wt. of Ba(OH)_(2) "reacted")/("Total weight of sample") xx 100` = 1.28 % |
|
| 51377. |
20CC of hydro carbon were exploded with excess of oxygen. After explosion and cooling a contraction of 20cc was noted on addition of KOH another contraction of 40CC was noted. The molecular formula of hydrocarbon is |
|
Answer» `C_(2)H_(6)` `CxHY+(X+(y)/(4))O_(2)rarrxCO_(2)+(y)/(2)H_(2)O` `{:("1 mol",(x+(y)/(4))"mol","x mol"),("1 vol",(x+y//4)"vol","x vol"),(20C C,20(x+(y)/(4))C C,20xC C):}` `CO_(2)` PRODUCED = contraction on addition of KOH = 40 CC implies 20x = 40 implies x = 2 `[20C C+20(x+(y)/(4))C C]-20xC C=30C C` `y=2` Formula of hydrocarbon =`C_(2)H_(2)` |
|
| 51378. |
2.0g of a metallic carbonate on decomposition gave 1.5g of metallic oxide. The equivalent mass of metal is |
|
Answer» 58 |
|
| 51379. |
200cc of ozone diffused in 15 min through a porous membrane. How much time does 150cc of oxygen take to difffuse, under similar conditions ? |
|
Answer» Solution :`(V_(O_3) xx t_(O_2))/( V_(O_2) xx t_(o_3)) = sqrt((M_(o_2))/(M_(O_3)))= >( 200 xx t_(o_2))/( 150 xx 15 ) = sqrt((32)/(48))` `t_(O_2) = ( 15 xx 150 )/(200) =sqrt((32)/(48))=9.135 ` MIN |
|
| 51380. |
200 mL of water is added to 500 mL of 0.2 M solution. What is the molarity of the diluted solution ? |
|
Answer» 0.5010 M `0.2Mxx500-=M_(2)xx700` `M_(2)=((0.2M)xx500)/(700)=0.1428M` |
|
| 51381. |
200ml of pure oxygen is subjected to electric discharge, 15% of oxygen is converted into ozone. The volume of ozonized oxygen is |
| Answer» ANSWER :C | |
| 51382. |
200 ml of O_2 gas maintained at 700mm pressure and 250ml of N_2 gas maintained at 720mm pressure are put together in one litre flask. If the temperature is kept constant, the final pressure of the mixture in mm is |
| Answer» Answer :B | |
| 51383. |
200 mL of a solution of mixture of NaOH and Na_(2)CO_(3) was first titrated with phenolphthalein and N//10HCl. 17.5 mL of HCl was required for the end point. After this methyl orange was added and 2.5 mL of same HCl was required for next end point. Find out amounts of NaOOH and Na_(2)CO_(3) in mixture. |
|
Answer» `Na_(2)CO_(3)=0.0265 g per 200 mL`; |
|
| 51384. |
200 ml of an aqueous solution of a protein contains 1.26 g of protein. At 300 K, the osmotic pressure of this solution is found to be2.52xx 10^(-3)bar.The molar mass of protein will be (R=0.083 Lbar mol ^(-1) K^(-1)) |
|
Answer» `62.22` KG `mol^(-1)` `pi = (W)/(M xxV) xx RT` ` therefore M = (WRT)/(PIV) = (1.26 xx 0.083 xx 300)/(2.52 xx 10 ^(-3) xx 0.2)= 62.22 kg mol ^(-1)` |
|
| 51385. |
200 mL of 3 N HCl were mixed with 200 mL of 6 N H_(2)SO_(4) solution. The final normality of H_(2)SO_(4) in the resultant solution will be: |
|
Answer» 9 N |
|
| 51386. |
20.0 L of 0.2 M weak acid (pK_(a)=5.0) is titrated against 0.2 M strong base. What is the pH at the equivalence point ? |
|
Answer» `5.0` |
|
| 51387. |
200 joules of heatwas supplied to a system at constant volume. It resulted in the increase in temperature of the system from 298 to 323 K. What is the change in internal energy of the system ? |
|
Answer» 400 J `w=-PDeltaV=0""DeltaU=q=200J` |
|
| 51388. |
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and8.0 g magnesium oxide. What will be the percentage of purity of magnesium carbonate in the sample? |
|
Answer» 60 84 g of `MgCO_(3)`gives 40 g of MgO. (100% purity) 20g of `MgCO_(3)` will give `=(40xx20)/84=9.52g` 9.52g of MgO is given by 100% pure `MgCO_(3)`. 8.0 g of MgO will be given by=`(100xx8)/9.52=84.03%` |
|
| 51389. |
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample ? (At. Wt of Mg = 24) |
|
Answer» 96 84 g of `MgCO_(3)` yield upon heating MgO = 40 g 20 g of `MgCO_(3)` yield upon heating `MgO=((40g)xx(20g))/((84g))` `=9.52g` Weight of MgO ACTUALLY formed = 8.0g % purity of `MgCO_(3)=((8.0g))/((9.52g))xx100=84%`. |
|
| 51390. |
20 volume. of H_(2)O_(2) is equal to |
|
Answer» `20% H_(2)O_(2)` by MASS |
|
| 51391. |
20 volume H_2O_2 Solution has a strength of about |
|
Answer» `30%` `THEREFORE` 1 litre `O_2` at N.T.P. obtained by `68/22.4` gm of `H_2O_2` `therefore` 20 litre `O_2` at N.T.P. obtained by `68/22.4xx20` gm of `H_2O_2` =60.71 gm of `H_2O_2` `therefore` 1000 ml `O_2` at N.T.P. obtained by =60.71 gm of `H_2O_2` `therefore` 100 ml `O_2` at N.T.P. obtained by `=60/1000xx100`=6.71% |
|
| 51392. |
20 % of N_(2)O_(4) " molecules are disociated in a sample of a gas at " 27^(@)C and 760 " torr . Calculate the density of the equilibrium mixture ". |
|
Answer» Solution :` {:(,N_(2)O_(4)(g),hArr,2NO_(2)(g),),("Intial",1 " mole",,,),("At.eqm.",1-0*2 = 0*8" mole",,0*4 "mole,",Total = 1*2 " moles" ):}` If V is the volume of the vapour PER mole, volume of vapour before dissociation = V ` " Hence density " (D) propto1/V ` But density after dissociation `D= (" Mol.wt.of " N_(2)O_(4))/2 = 92/2=46"" ("Theoretical density")` Volume after dissociation = `1*2` V ` :. "Density (d)" propto 1/(1*2 V) ` ` :.D/d = 1/V xx 1*2V =1*2OR d=D/(1*2)= 46/(1*2)= 38*3` Alternatively, USE theformula directly, ` alpha = (D-d)/d` |
|
| 51393. |
20 ml of the solution containg Na_(2)CO_(3) and NaHCO_(3) is titrated with 0.1MHCI using Phenolphthalein indicator the end point was 10ml. 20ml of the same solution is titrated with 0.1M HCI, the end point was 25ml with Methylorange indicaor from the begining. NaHCO_(3)+HCl rarr NaCl +H_(2)O +CO_(2) Na_(2)CO_(3)+2HCl rarr 2NaCl +CO_(2) +H_(2)O What amount of NaOHis required to convertNaHCO_(3) to Na_(2)CO_(3) in 1 litre of solution |
|
Answer» `2g` 84g of `NaHCO_(3)` requires 40g of `NaOH` `2.1g` of `NaHCO_(3)` requires? `= (40)/(84) xx 2.1 = 1g` |
|
| 51394. |
20 mL of x M HCl neutralises 5 mL of 0.2 M Na_(2)CO_(3) solution to phenolphthalein end-point. The value of x is |
|
Answer» `0.167M` EQ. `HCl=(1)/(2)` eq. `Na_(2)CO_(3)` `20XX X xx1=(1)/(2)xx5xx0.2xx2impliesx=0.05` |
|
| 51395. |
20 ml of the solution containg Na_(2)CO_(3) and NaHCO_(3) is titrated with 0.1MHCI using Phenolphthalein indicator the end point was 10ml. 20ml of the same solution is titrated with 0.1M HCI, the end point was 25ml with Methylorange indicaor from the begining. NaHCO_(3)+HCl rarr NaCl +H_(2)O +CO_(2) Na_(2)CO_(3)+2HCl rarr 2NaCl +CO_(2) +H_(2)O What is amount of NaHCO_(3) present in 1 litre of solution |
|
Answer» `2.1g` `x +y = 25 XX 0.1 = 2.5, y = 2.5 - 2 = 0.5` Wt of `NaHCO_(3)` in 20ml `=(0.5)/(1000) xx 84 = 0.042g` wt of `NaHCO_(3)` in 1 lit `=(0.042)/(20) xx 1000 = 2.1g` |
|
| 51396. |
20 ml of nitric oxide combines with 10 ml of oxygen at STP to give NO_(2). The final volume will be |
|
Answer» 30ml |
|
| 51397. |
20 mL of HCl having a certain normality neutralises exactly 1.0 g CaCO_(3) . The normality of acid is |
|
Answer» 0.5 N 100 g of `CaCO_(3)` shall be NEUTRALISED by `(73)/(100)=0.73 g ` HCl `therefore 20` mL of HCl has HCl in equivalent `=(0.73)/(36.5)` Hence Normality `=(0.73)/(36.5)XX(1000)/(20)=1.0 N` |
|
| 51398. |
20 mL of hydrogen measured at 15^(@)C are heated to 35^(@)C. What is the new volume at the same pressure ? |
|
Answer» Solution :`{:("Given CONDITIONS","Final Conditions"),(V_(1)=20 mL,V_(2)=?mL),(T_(1)=15+273=288 K ,T_(2)=35+273=308 K):}` By applying Charles' LAW,`(V_(2))/(308)=(20)/(288)" or " V_(2)=(20)/(288)xx308=21.38` Volume of hydrogen gas at `35^(@)C=21.38 mL` |
|
| 51399. |
20 ml of H_(2)O_(2) after acidification with dil H_(2)SO_(4), required 30 ml of N/2 KMnO_(4) for complete oxidation. Calculate the % of H_(2)O_(2) in gr/lit. |
|
Answer» 10.75 g/lit `(W times 1000)/(34//2)=1/2 times 30` `W=1/2 times (300 times 17)/1000=0.255 gr//20ml` `N_(H_(2)O_(2))=0.255/17 times 1000/20=0.75` STRENGTH `=0.75 times 17=12.75` gr/lit |
|
| 51400. |
20 mL of a solution of H_2SO_4 neutralises 21.2 mL of 30% solution (w/v) of Na_2CO_3. How much water should be added to each 100 mL of the solution to bring down its strength to decinormal ? |
|
Answer» `underset(105.99 g)(Na_(2)CO_(3)) + underset(98.076 g)(H_(2)SO_(4)) to Na_(2)SO_(4) + H_(2)O + CO_(2)` The amount of `Na_2CO_3` present in 21.2 mL solution. `=3/100 xx 21.2 = 0.63 g` `therefore 105.99 g` of `Na_(2)CO_(3)` react with `H_(2)SO_(4) = 98.076 g` `therefore 0.636 g` of `Na_(2)CO_(3)` will react with `H_(2)SO_(4)` `=98.076/105.99 xx 0.636 = 0.588 g` This much `H_(2)SO_(4)` is present in 20 mL. `therefore W=(NEV)/1000` `therefore 0.588 = (N xx 49.038 xx 20)/1000` (Eq. wt. of `H_(2)SO_(4) = (98.076)/2 = 49.038`) `therefore N=0.599 = 0.6` Therefore, the normality of the given `H_2SO_4` solution is 0.6 N. Suppose, v mL of water are REQUIRED to be added to 100 mL of it to make it decinormal `N/10` `therefore 0.6 xx 100 = 1/10 xx (100 + v)` which GIVES v = 500 mL Hence, 500 mL of water should be added to each 100 mL of the solution of `H_2SO_4` to bring down its strength to decinormal. |
|