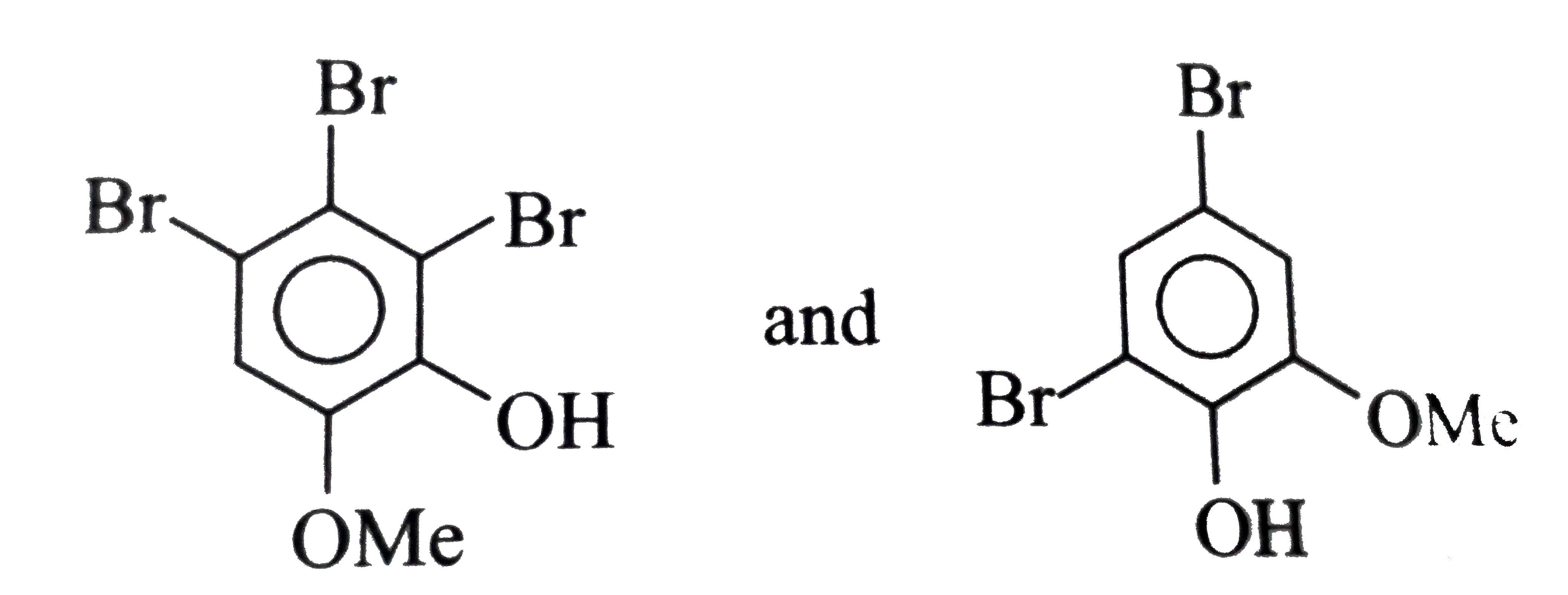Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 1451. |
The vapour pressure of water at 20^(@)C is 17.5 mm Hg. If 18 g of glucose (C_(6)H_(12)O_(6))is added 178.2 g of water at 20^(@)C, the vapour pressure of the resulting solution will be : |
|
Answer» <P>17.325 MM Hg `P_(A)=P_(A)^(@)X_(A)` `X_(A)=n_(A)/(n_(A)+n_(B))=((178.2)/18)/((188.2)/18+18/180)` `=(9.9)/(9.94)=0.99` `P_(A)=(17.5mm)xx0.99=17.32 mm` |
|
| 1452. |
Which of the following alkali metal carbonates is the most stable towards decomposition ? |
|
Answer» `Li_2CO_3` |
|
| 1453. |
Which of the following will show schottky defect |
|
Answer» `CaF_(2)` |
|
| 1454. |
Toluene is reacted with Cl_(2) in the presence of Fe Cl_(3) give p-chlorotoluene as the major product because the methyl group is 1. p-directing 2. m-directing 3. activatingh the ring by hyper conjugation 4. deactivate the ring |
|
Answer» 1 |
|
| 1455. |
What weight of ""^(14)C (t_((1)/(2))=5760 years) will make one curie of it ? [one curie gives 3.7 xx 10^(10) disintegrations/second (dps)] |
|
Answer» Solution :Let the weight of `""^(14)C` be w gram Number of `""^(14)C` nuclei= mole `XX` Av. CONSTANT `=(w)/(14) xx 6.022 xx 10^(23)` and `t_((1)/(2))` in second `=5760 xx 365 xx 24 xx 60xx 60` Now, we have, `-(d(N))/(dt)= lamda (N)= (0.6932)/(t_((1)/(2))) xx N` `3.7 xx 10^(10)= (0.6932)/(5760 xx 365 xx 24 xx 60 xx 60)xx (w)/(14) xx 6.022 xx 10^(23)` w= 0.225g |
|
| 1456. |
What is kraft temperature? |
| Answer» Solution :The FORMATION of micelles TAKES place only above a particular TEMPERATURE called KRAFT temperature | |
| 1457. |
Which of the following terms indicates to the arrangement of different protein subunits in a multiprotein complex ? |
|
Answer» PRIMARY structure |
|
| 1458. |
Which of the following statements are in accordance with the Arrhenius equation ? |
|
Answer» Rate of a REACTION increases with increase in temperature |
|
| 1459. |
What is the potential of a half-cell consisting of zinc electrode in "0.01 M ZnSO_(4) solution 25^(@)C. E^(@) = 0.763 V. |
|
Answer» Solution :The half-cell reaction is `Znrarr Zn^(2+)+2e^(-)` The Nernst equation for the oxidation half-cell reaction is `E=E^(@)-(0.0591)/(n)log[Zn^(2+)]` The number of electrons transferred n = `2 and E^(@) = 0.763 V` SUBSTITUTING these values in the Nernst equation we have `E=0.763-(0.0591)/(2)(-2)` `=0.763+0.0591=0.8221V` CALCULATION of Cell POTENTIAL The Nernst equation is applicable to cell potentials as well. THUS, `E_("cell")=E_("cell")^(@)-(0.0591)/(n)logK` K is the equilibrium constant of the redox cell reaction. |
|
| 1460. |
Why aromatic amines possess low basicity ? |
| Answer» Solution :Due to PRESENCE of electron drawing phenyl GROUP aromatic amines are LESS BASIC in nature. The lone pair to electron is ENGAGED in resonance. | |
| 1461. |
What would be the mass as well as molarity if the half cell reaction . 2BrO_(3)^(-) + 12H^(+) + 10e^(-) rarrBr_(2) + 6 H_(2)O |
|
Answer» |
|
| 1462. |
The standard heats of formation of CH_(4), H_(2)O and CH_(3)OHare -76, -242 and -266 kJ/mole respectively. The enthalpyCH_(3)OH_((l))+H_(2(g))rarrCH_(4(g))+H_(2)O_((l)) |
|
Answer» `-4 kJ//"MOLE"` `DeltaH=(-242-76)-(-266+0)=-52 kJ`. |
|
| 1463. |
Which of the following is the buffer solution of strong acidic nature |
|
Answer» `HCOOH + HCOO^(-)` |
|
| 1464. |
Which of the following is notna optically activecompound? |
|
Answer» Alanine |
|
| 1465. |
When primary amides are treated with P_(2)O_(5), they give : |
|
Answer» Amines |
|
| 1466. |
What are fuel cells? |
| Answer» Solution :A galvanic cell that are desgined to convert, the ENERGY of FUEL like HYDROGEN, methane, methanol ETC. into electrical energy. | |
| 1468. |
The treatment of diseases by using drugs is known as |
|
Answer» physiotherapy |
|
| 1470. |
The value of one Faraday is |
|
Answer» `95500C mol^-1` |
|
| 1471. |
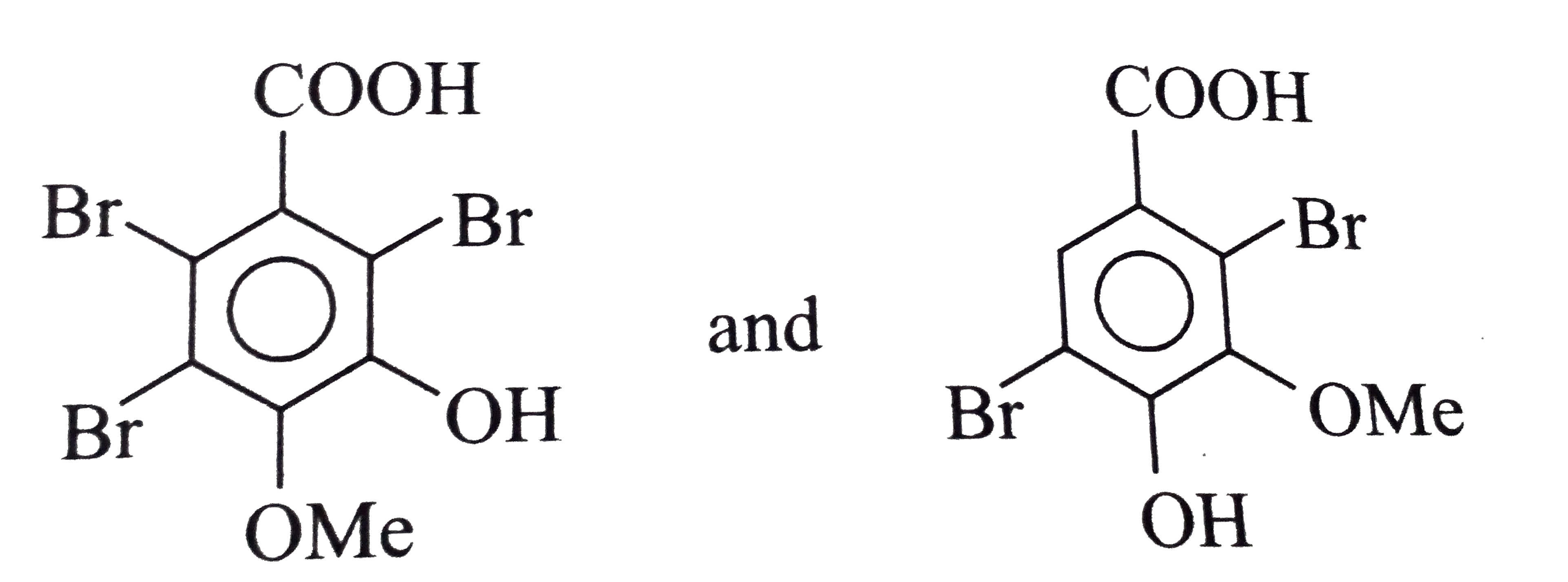
Which of the following statements is/are correct about the given reaction ? (A) (B)(C_(7)H_(5)Br_(3)O_(2)) (C ) (D)(C_(7)H_(6)Br_(2)O_(2)) |
|
Answer» The PRODUCTS (B) and (D), respectively, are: <BR> |
|
| 1472. |
What are surfactants. Gove one point of distinction between soaps and detergents. |
| Answer» | |
| 1473. |
The reduction of CH_3CN to CH_3CH_2NH_2 is called : |
|
Answer» ROSENMUND REDUCTION |
|
| 1474. |
What is the weight of oxygen that is required for the complete combustion of 2.8 kg of ethylene |
|
Answer» 9.6 kg |
|
| 1475. |
The unit of rate constant for a zero order reaction is |
|
Answer» litre `sec^(-1)` |
|
| 1476. |
The theory of electrolytic dissociation was given by |
|
Answer» DALTON |
|
| 1477. |
Which of the following is an example of cationic detergent? |
|
Answer» SODIUM lauryl sulphate |
|
| 1478. |
What happens when: (write chemical equations only ) (a) Anhydrous AlCl_(3) is exposed to atmosphere. (b) AlCl_(3) dissolves in aqueous sodium hydroxide (excess) |
|
Answer» Solution :(a)`AlCl_(3)+3H_(2)OtoAL(OH)_(3)+3HCl , (HCl` fumes in air). (B)`AlCl_(3)+3NaOH to AL(OH)_(3)darr+3NaCl` `Al(OH)_(3)+OH^(-)to[Al(OH)_(4)]^(-)` |
|
| 1479. |
Which of the following can give cannizzaro's reaction with a base |
|
Answer» `CH_(3)(CH_(2))_(2)CHO` |
|
| 1480. |
Which two sets of reactants best represent the amphoteric character of Zn(OH)_(2)? Set1: Zn(OH)_(2)(s) and OH^(-)(aq) Set 2:Zn(OH)_(2)(s) and H_(2)O(l) Set 3:Zn(OH)_(2)(s) and H^(+)(aq) Set 4 : Zn(OH)_(2)(s) and NH_(3)(aq) |
|
Answer» 1 and 2 `underset("Base")(Zn(OH)_(2))+underset("Acid")(2H^(+)) to underset("Salt")(Zn^(2+)+underset("Water")(2H_(2)O)` |
|
| 1481. |
Wrong statement about O_(2)F_(2) is |
|
Answer» It is non-planar molecule |
|
| 1482. |
Which of the following cannot act as an oxidising agent ? |
| Answer» Answer :A | |
| 1483. |
Which of the following is useful as a food preservative |
|
Answer» SALTS of sorbic acid |
|
| 1484. |
The units of conductivity are |
|
Answer» `ohm^-1` |
|
| 1485. |
Which of the following are biopolymers? |
|
Answer» NUCLEIC ACIDS |
|
| 1486. |
What is the reagent used for dehydrohalogenation of an alkyl halide? |
|
Answer» Solution :ALCOHOLIC KOH. for EXAMPLE, `underset("Bromoethane")(CH_(3)-CH_(2)-Br) underset(("Dehydrohalogenation"))OVERSET("Alc KOH, "Delta)to underset("Ethene")(CH_(2)=CH_(2))+KB r+H_(2)O` |
|
| 1487. |
Treatment of acetaldehyde with ethyl magnesium bromide and subsequent hydrolysis gives: |
|
Answer» 1-butanol |
|
| 1488. |
Which of the following is used as a local anaesthetic agent ? |
|
Answer» Cocaine |
|
| 1489. |
Which is a stronger reducing agent, SbH_(3) or BiH_(3) and Why? |
| Answer» SOLUTION :`BiH_(3)`, because the STABILITY of hydrides decreases on MOVING from `SbH_(3)` to `BiH_(3)`. | |
| 1490. |
Which component of strach in branched polymer of a glucose and insolublein water . |
| Answer» SOLUTION :AMYLOPECTIN. | |
| 1491. |
Which out of helium and neon would adsorb on the surface of charcoal more readily and why? |
| Answer» Solution :Neon would adsorb more READILY DUE to its greater molecular mass and SURFACE area and hence greater VAN der WAALS. forces. | |
| 1492. |
Which of the following aqueous solution has the highest boiling point |
|
Answer» `0.1M KNO_(3)` |
|
| 1493. |
Which of the following species can act as strongest base |
|
Answer» `""^(ODOT)OH` |
|
| 1494. |
Which one of the following is the most basic? |
|
Answer» Ammonia |
|
| 1495. |
Which solution will show highest resistance during the passage of current |
|
Answer» 0.05 N NaCl |
|
| 1496. |
Which of the following statements are ture about semi-conductors ? |
|
Answer» Silicon doped with ELECTRON rich impurity is a p-type semi-conductor (a) Silicon doped with electron rich impurity is an n-type semiconductor. Therefore, STAEMENT (a) is false. ltbRgt (b) The conductivity of n-type semi-conductors increases DUE to extraelectrons and not because of electron valency. Therefore statement (b) is false. |
|
| 1497. |
The solublity of AgCl is formed when equal volumes of the following are mixed. [K_(sp) for AgCl = 10^(-10)] |
|
Answer» `2.0 xx 10^(-5) M` For AgCl `AgCl hArr AG^(+) + Cl^(-)` `K_(sp) = [Ag^(+)][Cl^(-)]` `4.0 xx 10^(-10) = [S][S+0.08]` `4.0 xx 10^(-10) = S^(2) + S xx 0.08 [S^(2) lt lt lt 1]` `4.0 xx 10^(-10) = S xx 0.08` `S = (4.0 xx 10^(-10))/(0.08) = 0.5 xx 10^(-8) = 5 xx 10^(-9)`. |
|
| 1498. |
Which of the following reactions proceed at low pressure |
|
Answer» `N_(2)+3H_(2)hArr2NH_(3)` |
|
| 1499. |
When a rod of metal A is dipped in an aqueous solution of metal B (concentration of B^(2+) ion being 1M) at 25^(@)C, the standard electrodepotentials are A^(2+)//A=-0.76volts, B^(2+)//B=+0.34 volts |
|
Answer» A will gradually dissolve `A+B^(2+)toA^(2+)+B`. |
|
| 1500. |
The two strands in DNA are not identical but complementary. Explain. |
| Answer» Solution :Base PAIRING RULE is followed, A = T and G `-= C`. | |