Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 1501. |
Which of the following is not chemical twin? |
|
Answer» Mo-W |
|
| 1502. |
Which one of the following pairs is isostructural (i.e., having the same shape and hybridization) ? |
|
Answer» `[BF_(4)^(-)`and`NH_(4)^(+)]` |
|
| 1503. |
Whatarehormones ? Give examples. |
|
Answer» Solution :HORMONE isorganicsubstance thatis screted byon TISSUE. It isan intercellularsignalingmolecule . Virtually every procoseis a complex organism is regulated byone or more hormones . EXMPLE ,insulin , epinephrine , estrogen, androgent etc. |
|
| 1505. |
Which can be prepared by Kolbe's electrolysis? |
|
Answer» `C_2H_6` |
|
| 1506. |
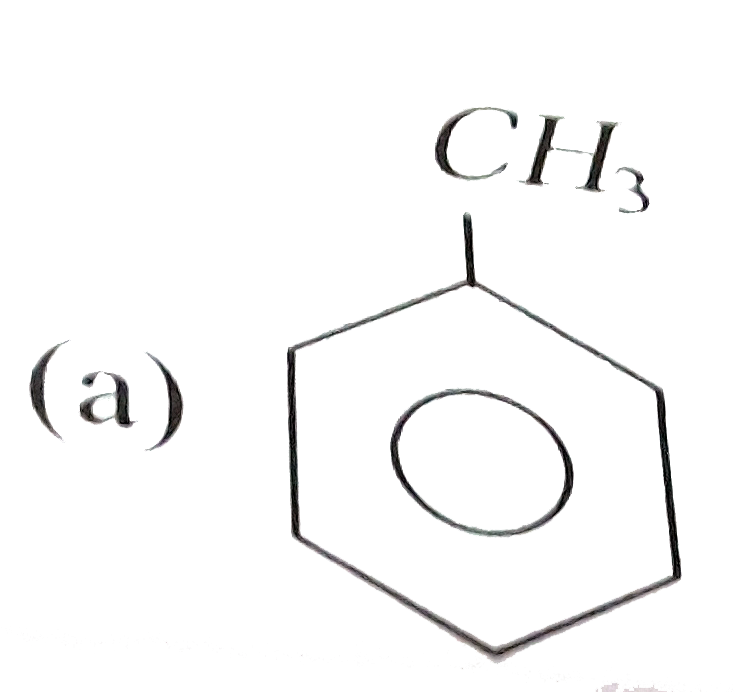
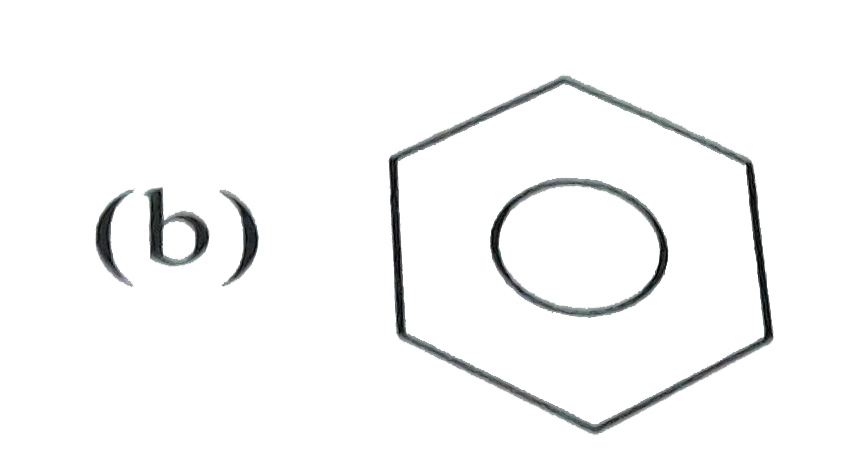
Which of the following will be easily nitrated |
|
Answer»
|
|
| 1507. |
The substance used in the thermite process for reducing metal oxide is ________. |
| Answer» Answer :A | |
| 1508. |
Which one of the following statement is not true |
|
Answer» Clean water would have a BOD value of 5 ppm. |
|
| 1509. |
The yellow coloured solution of chromate salt changes to orange colour on acidification due to the formation of : |
|
Answer» `CR^(3+)` |
|
| 1510. |
What is the name of most effective sulpha drug ? |
| Answer» SOLUTION :SULPHANILAMIDE and SULPHADIAZINE . | |
| 1511. |
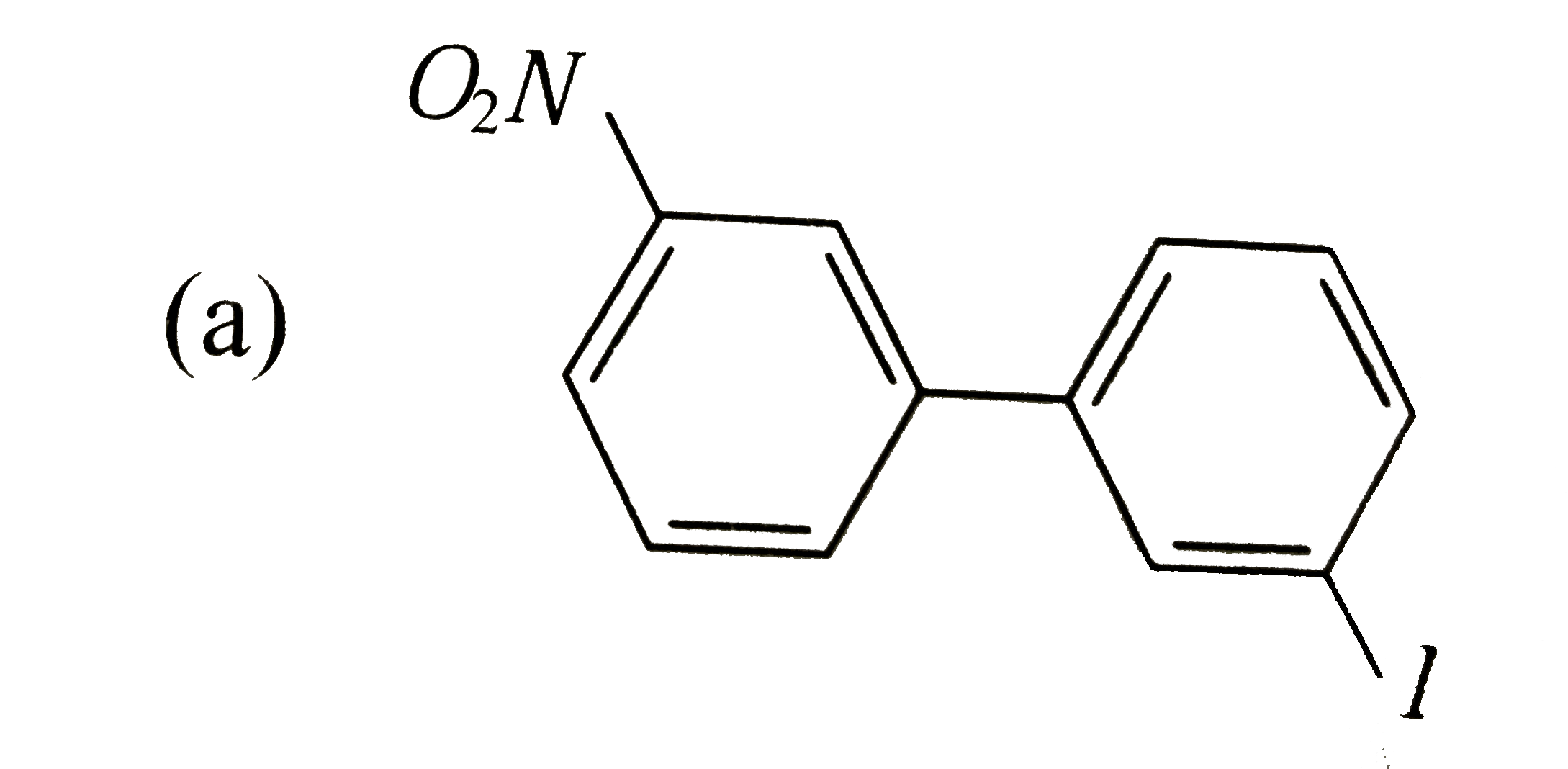
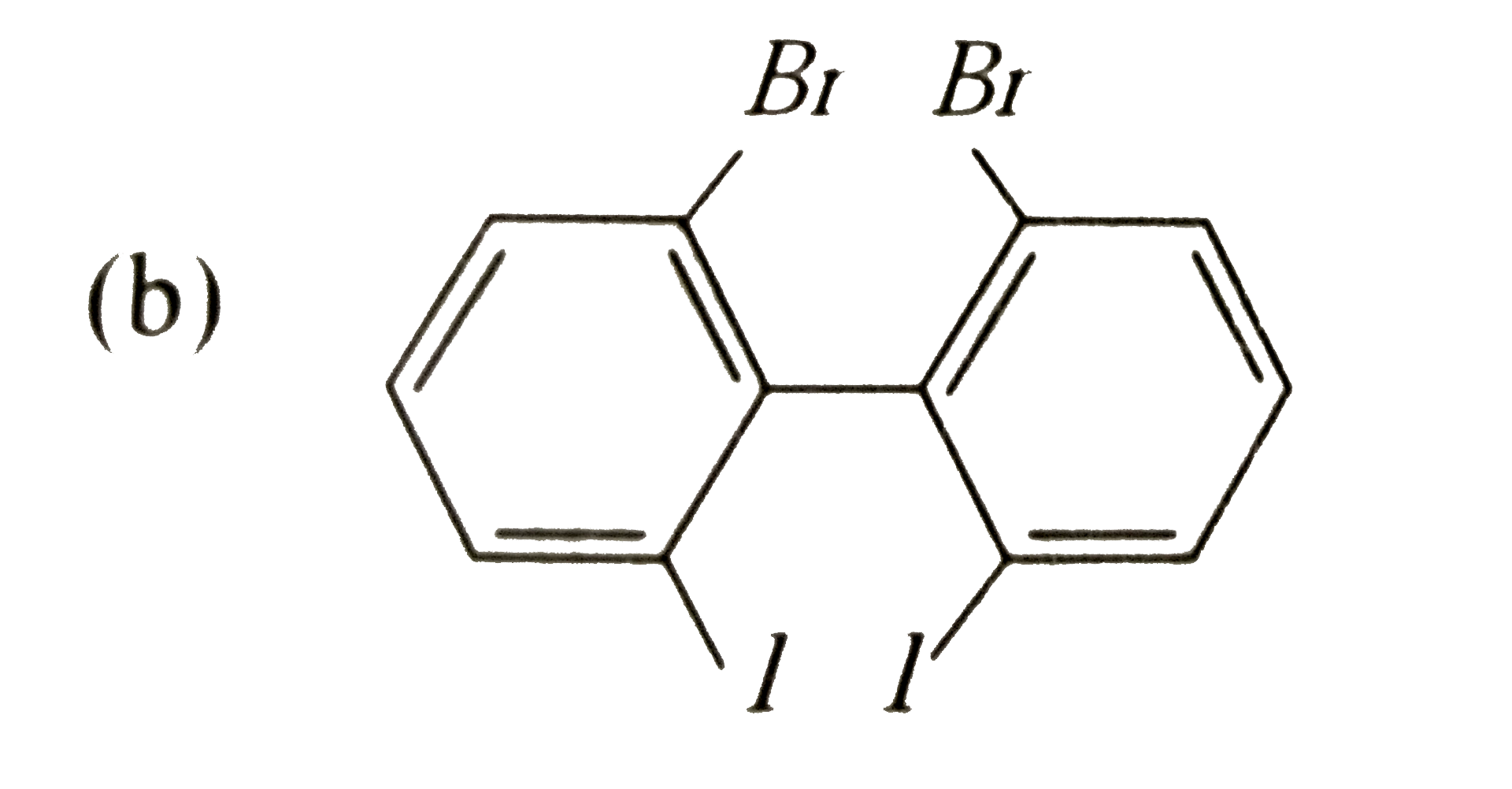
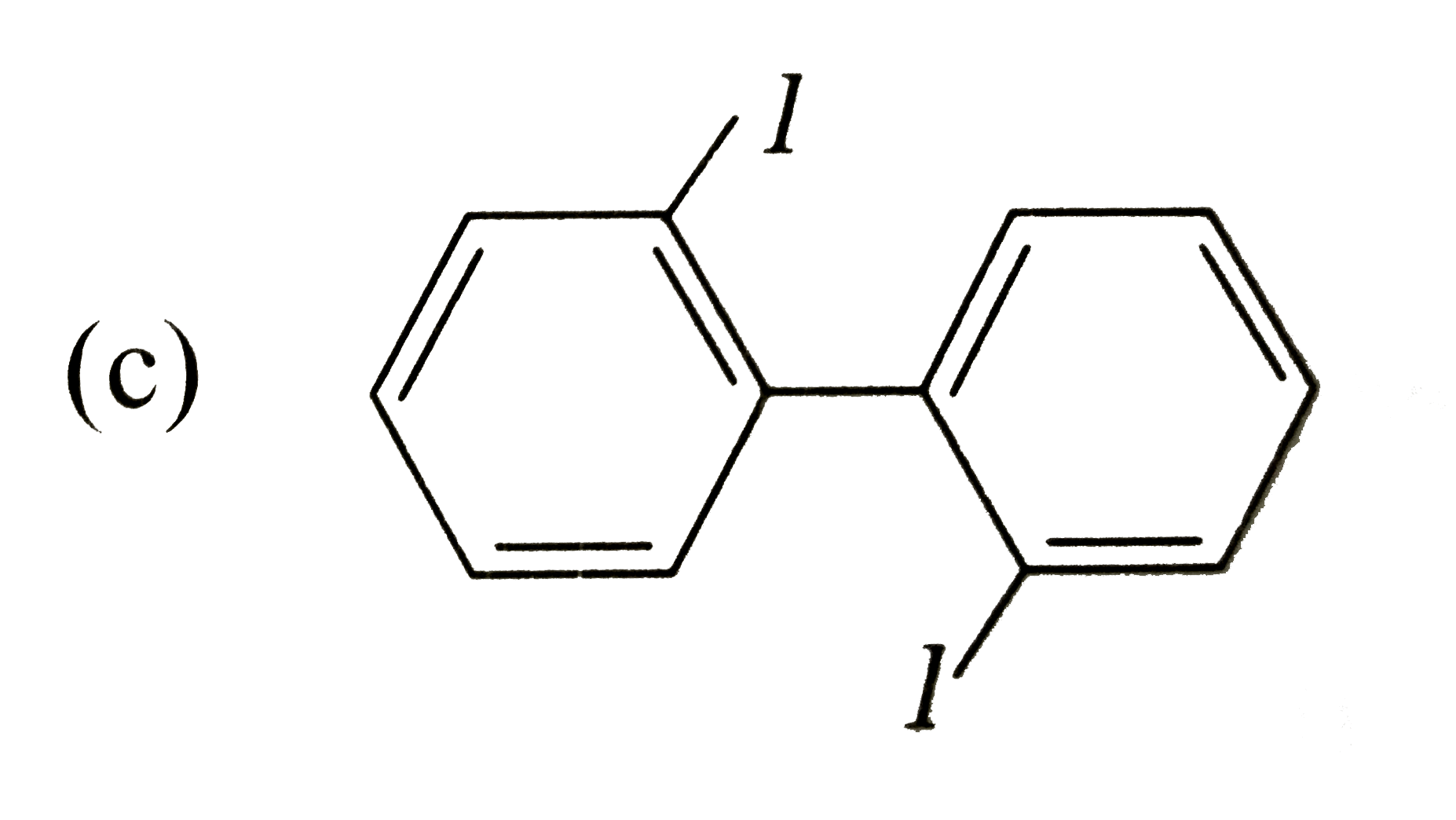
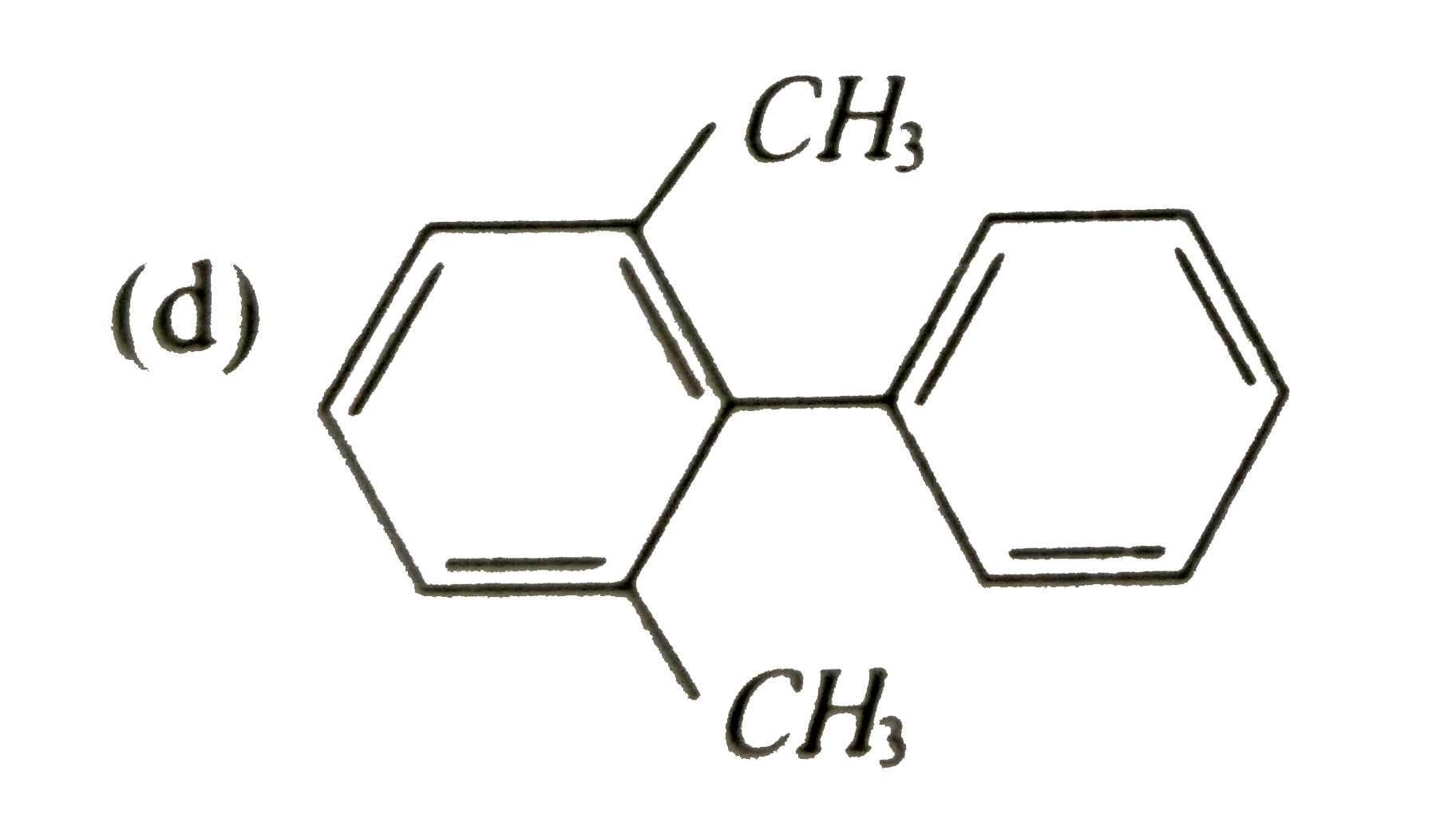
Which of the following biphenyls is optically active? |
|
Answer»
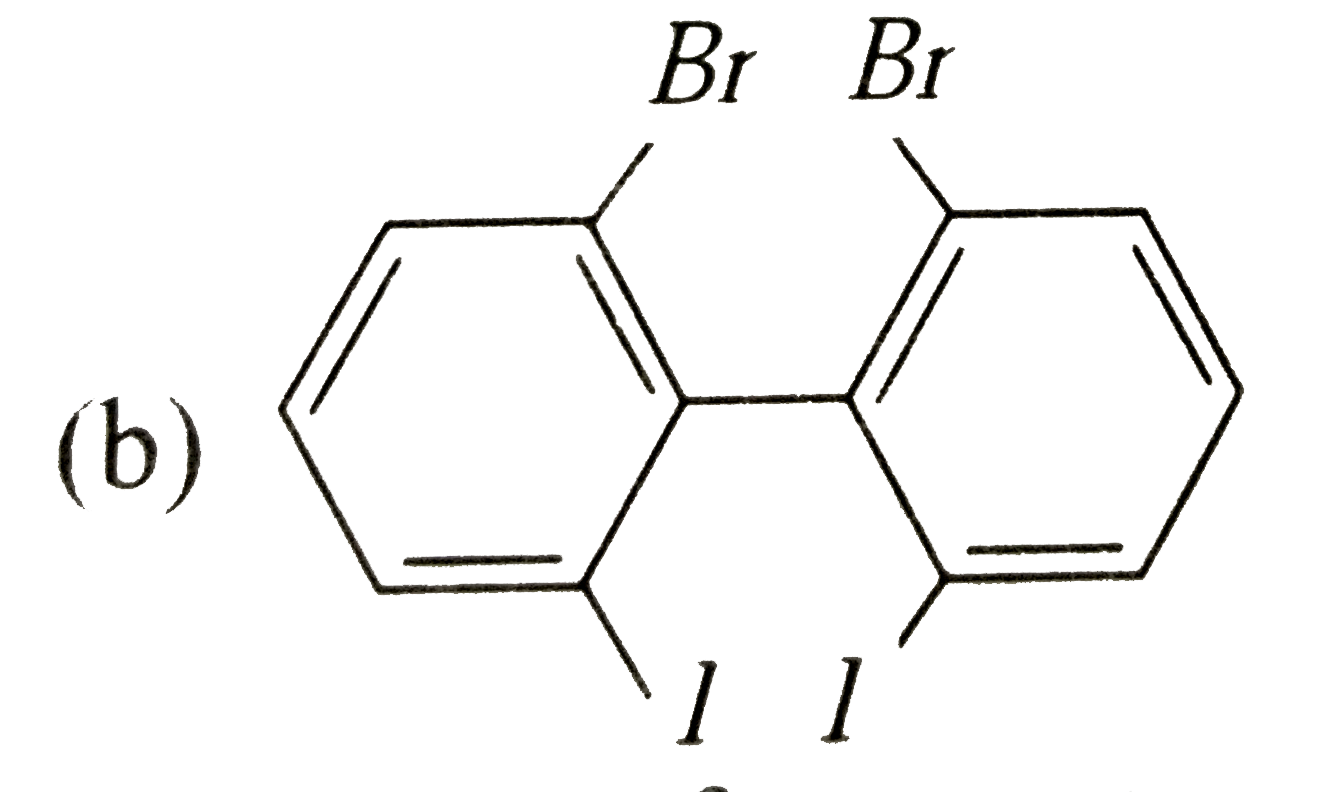 is optically ACTIVE DUE to absence of plane of symmetry and CENTER of symmetry. is optically ACTIVE DUE to absence of plane of symmetry and CENTER of symmetry.
|
|
| 1512. |
Which is a monosaccharide among the following? |
|
Answer» SUCROSE |
|
| 1513. |
The S - S - S bond angle in S_(8) molecule is |
|
Answer» `109.5 ^(@)` |
|
| 1514. |
Which of the following amorphous solid is used as photovoltaic material for conversion of sunlight into electricity? |
|
Answer» Quartz glass |
|
| 1515. |
Which one is more stable and why? |
|
Answer» |
|
| 1516. |
What is the difference between a collodial sol, gel and emulsion ? |
| Answer» Solution :In a COLLOIDAL sol, the dispeersed phase is a soolid and the DISPERSION mdium is a LIQUID. In a gel, it is opposite. In an EMULSION, both the dispersed phase and dispersion medium are liquids. | |
| 1518. |
Which of the following acts as stimulator? |
|
Answer» VITAMIN |
|
| 1519. |
Write thechemical reactionfor reactions involved in synthesis of Bakelite . |
Answer» SOLUTION :
|
|
| 1520. |
Write the IUPAC name of the compound : CH_(3)-underset(CH_(3))underset("|")"CH"-CO-underset(CH_(3))underset("|")"CH"-CH_(3) |
| Answer» SOLUTION :2, 4 - Dimethylpentan -3- ONE. | |
| 1521. |
Which of the following is a gas: |
|
Answer» METHANE thiol |
|
| 1522. |
What is Tollen's reagent? Write one usefulness of this reagent. |
|
Answer» Solution :Tollens reagent is AMMONICAL silver NITRATE solution `[Ag(NH_(3))_(2)]OH` It is used to DISTINGUISH between aldehydes and ketones. |
|
| 1524. |
Which one of the following reaction occurs at the cathode |
|
Answer» `2OH^(-) RARR H_(2) O + 1/2 O_(2) + 2e^(-)` |
|
| 1525. |
The test used to distinguish alcohols from one another is known as |
|
Answer» Hinsberg's test |
|
| 1526. |
What are tranqilizers ? |
|
Answer» Solution :Class of CHEMICALCOMPOUNDS USED for the treatment of STRESS. OR Any suitable statement Detailed Answer : Tranquilizers are the DRUGS used to reduce stress, mild and severs mental disease. |
|
| 1527. |
Write the name of the compound with R//S-designation obtained when (R)-2 bromocatane is subject to alkaline hydroysis (S_(N)2). |
| Answer» SOLUTION :(S)-2-octanol. | |
| 1528. |
Which step is not involved in hydrometallurgical process ? |
|
Answer» `Cu_(2)S + 2Cu_(2)O rarr 6 CU + SO_(2)` |
|
| 1529. |
Which yields isopropyl methyl ether with little or no by-products? |
|
Answer» `(CH_(3))_(2)CHO^(-)Na^(+)+CH_(3)Irarr` |
|
| 1530. |
The total energy of molecules is divided equally amongst the various degrees of freedom of a molecule. The distribution of kinetic energy along x, y, z axis are E_(K_(x)), E_(K_(y)), E_(K_(z)) Total K.e =E_(K_(x)) + E_(K_(y)) + E_(K_(z)) Since the motion of molecule is equally probable in all the three directions, therefore E_(K_(x)) = E_(K_(y)) = E_(K_(z)) =1/3 E_(K) =1/3 xx 3/2 kT = 1/2 kT, where k =R/N_(A) = Botzman constant. K.E. = 1/2 kT per molecule or =1/2 RT per mole. In vibration motion, molecules possess both kinetic energy as well as potential energy. This means energy of vibration involves two degrees of fiuedom. Vibration energy =2 xx 1/2kT =2 xx 1/2RT [therefore two degrees of freedom per mole] If the gas molecules have n_(1) translational degrees of freedom, n_2 rotational degrees of freedom and n_(3) vibrational degrees of freedom, that total energy = n_(1)[(kT)/2] + n_(2) [(kT)/2] + n_(3) [(kT)/2] xx 2 Where 'n' is atomicity of gas. The rotational kinetic energy of H20 molecule is equal to |
|
Answer» `3/2 KT` |
|
| 1531. |
Which of the following sugars can be used to prepare glucose on a large scale ? |
|
Answer» Cellulose |
|
| 1532. |
Which one of the following is a cyclic oxoacid ? |
|
Answer» <P>`H_(4) P_2O_7` 
|
|
| 1533. |
Two reactions A rarr Products and Brarr products have rate constants k_(A) and k_(B) respectively at temperature T and activation energies E_(A) and E_(B) respectively . If k_(A) gt K_(B) and E_(A) lt E_(B) and assuming that A for both the reactions is same then : |
|
Answer» at higher TEMPERATURE `k_(A)` WILLBE greater than `k_(B)` |
|
| 1534. |
Which compound is formed when chloroform is exposed to air in presence of light? |
| Answer» SOLUTION :PHOSGENE | |
| 1535. |
What is piback bonding? |
|
Answer» SOLUTION :(i)In metal carbonyls , the bond between metal atom and the carbonyl ligand consists of two components (ii) The first component is an electron pair donation from the carbon atom of carbonyl ligand into a vacant d-orbital of central metal atom. (ii) This electron pair donation forms ` M overset(sigma" bond ")leftarrowCO ` sigma bond. This sigma bond formation increases th electron dentisity in metal d ORBITALS and makes the metal electron RICH . (IV) In order to compensate for this increased electron density ,a field metal d-orbital interacts with the empty `pi*` orbital on the carbonyl ligand and transfers the added electron density back to the ligand. |
|
| 1536. |
Which of the following has lowest magnetic moment ? |
|
Answer» `CU^(2+)` |
|
| 1537. |
Which of the following ions can be separated by using NH_(4)CI and NH_(4)OH? |
|
Answer» `Fe^(+3)` and `Cr^(+3)` |
|
| 1538. |
What is the approximate mass of the atmosphere of the earth? Assume the radius of the earth to be 6370 km. |
|
Answer» Solution :SURFACE are of the earth `=4pir^2` ATMOSPHERIC pressure = 1 atm = `101325 Pa` `5.27 xx 10^18 kg` |
|
| 1539. |
Which of the following serves as an indicator of atmospheric pollution |
|
Answer» Ferns |
|
| 1540. |
Write short note on the following : (i) Carbylamine reaction (ii) Diazotization (iii) Hoffmann's bromide reaction (iv) Coupling reaction |
Answer» Solution :(i) CARBYLAMINE reaction : When primary amine(aromatic of aliphatic) warned with chloroform and alc. KOH, isocyanides are formed which can be identified by their OFFENSIVE smell. This test is used to identifythe presence of primary amine or chloroform  . . (ii) Diazotization : When primary aromatic amine is treated with `NaNO_(2)` and HCI at 273-278 K, diazonium salt is obtained. This reaction is known as diazotization. Benzenediazonium chloride is a very important synthetic compound, which can be changed into heloarenes, phenol, cyanobenzene, BENZENE etc. 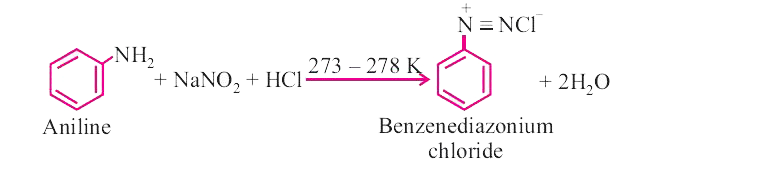 (III) Hoffmann.s bromide reaction : When any primary amide (alphatic or aromatic) is treated with bromine and alkali, it gives the amine with one less carbon atom.  This reaction is used to reduce one carbon atom from a compound. ## (iv) COUPLING reaction : When benzenediazonium chloride is treated with phenols or aromatic amines, azo dyes are produced in which diazo `(-N = N-)` group is retained. Coupling reactions generally take place at p-position of phenol or aromatic amines. 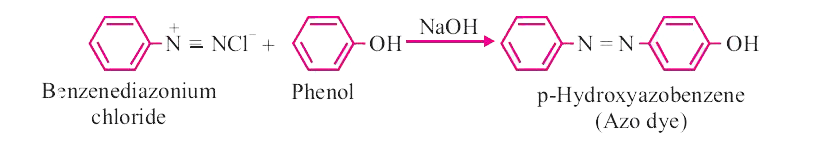 (v) Ammonolysis : Reaction of alkyl halides with ammonia is known as ammonolysis. Ammonolysis generally gives the mixture of `1^(@), 2^(@), 3^(@)` amines and quaternary ammonium salt. 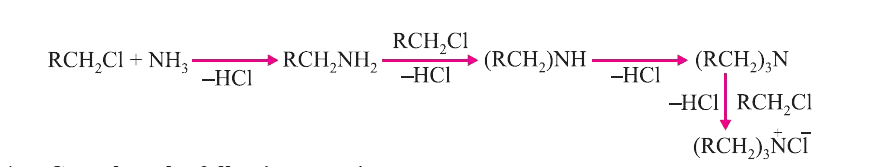
|
|
| 1541. |
Which of the following constitutes the genetic material of the cell ? |
|
Answer» NUCLEIC acids |
|
| 1542. |
Whichof the followingntiroalkanecan giveonlybluecolurednitroso- alkanewith HNO_(2) |
|
Answer» `CH_(3)-NO_(2)` 
|
|
| 1543. |
What is the reaction occuring at the anode in Duw's process for the extraction of sodium? |
|
Answer» `4OH^(-) to 2H_(2)O+O_(2)+4E^(-)` |
|
| 1544. |
The total number of lone pairs of electrons present in a S_8 molecule. |
|
Answer» 8 |
|
| 1545. |
The relative rates of reaction with concentrated H_(2)SO_(4) of the following is (I) (II) (III) |
|
Answer» `I GT II gt III` |
|
| 1547. |
Which of the following salt is alkaline in water |
|
Answer» `C Cl_(4)` |
|
| 1548. |
Write the essential conditions for geometrical isomerism. |
|
Answer» (1)Restricted rotation must present. (2) Two different groups must be present on both restricted atoms. (3) Groups responsible to SHOW geometrical isomerism must be NEARLY in the same plane. |
|
| 1549. |
Which of the following is not a sugar substituent? |
|
Answer» Sorbitol |
|
| 1550. |
Two elements A and B form compounds having molecular formula AB_(2) and AB_(4). When dissolved in 20 g of benzene (C_(6)H_(6)), 1 g of AB_(2) lowers the freezing point by 2.4 K whereas 1.0 g of AB_(4) lowers it by 1.3 K. The molal depression constant of benzene is "5.1 K kg mol"^(-1). Calculate atomic masses of A and B. |
|
Answer» Solution :Applying the formula, `M_(2)=(1000K_(F)w_(2))/(w_(1)xxDeltaT_(f))` `M_(AB_(2))=(1000xx5.1xx1)/(20xx2.3)"110.87 g mol"^(1),M_(AB_(4))= (1000xx5.1xx1)/(20xx1.3)= "196.15 g mol"^(-1)` Suppose atomic masses of A and B are 'a' and 'b' RESPECTIVELY. Then Molar mass of `AB_(2)=a+2b=110.87" g mol"^(-1)"...(i)"` Molar mass of `AB_(4)=a+4b="196.15 g mol"^(-1)"...(ii)"` EQN. (ii) - Eqn. (i) gives 2 b = 85.28 or b = 42.64 SUBSTITUTING in eqn. (i), we GET `a+2xx42.64=110.87 or a=25.59` Thus, Atomic mass of A = 25.59 u, `""` Atomic mass of B = 42.64 u |
|