Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 1601. |
Two weak acid solution HA_(1) and HA_(2) each with the same concentration and having pK_(a) values 3 and 5 are placed in contact with hydrogen electrode (1 atm, 25^(@)C) and are interconnected through salt bridge. Calculate the e.m.f. of the cell. |
|
Answer» Solution :The cell may be represented as `Pt|H_(2)(1atm)|HA_(2)||HA_(1)|H_(2)(1atm)|Pt` electrode REACTION expressed as reduction reaction is `H^(+)+e^(-)to(1)/(2)H_(2)` At ANODE: `E_((H^(+)//H_(2))_(2))=E_((H^(+)//H_(2))_(2))^(@)+0.059(pH)_(2)` At cathode: `E_((H^(+)//H_(2))_(1))=E_((H^(+)//H_(2))_(1))^(@)+0.059(pH)_(1)` But `[H^(+)]=Calpha=Csqrt((K_(a))/(C))=sqrt(K_(a)C)=(K_(a)C)^(1//2)` or `-LOG[H^(+)]=-(1)/(2)logK_(a)-(1)/(2)LOGC` `pH_(2)=(1)/(2)pK_(a)-(1)/(2)logC` `E_(cell)^(@)=E_((H^(+)//H_(2))_(1))^(@)-E_((H^(+)//H_(2))_(2))^(@)=0.059[(1)/(2)pK_(a_(2))-(1)/(2)pK_(a_(1))]=(0.0591)/(2)(5-3)` =0.059V |
|
| 1602. |
The resistance of 0.05M KCl solution at 25^@Cis 100 ohm in a cell whose cell constant is 0.3765cm^(-1). The specific conductivity of KCl solution is |
|
Answer» 0.004765 |
|
| 1603. |
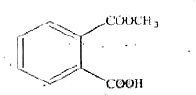
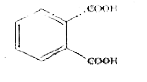
Total possible linkage isomers of K_(4)[Fe(CN)_(5)(NO_(2))] is : |
|
Answer» 12 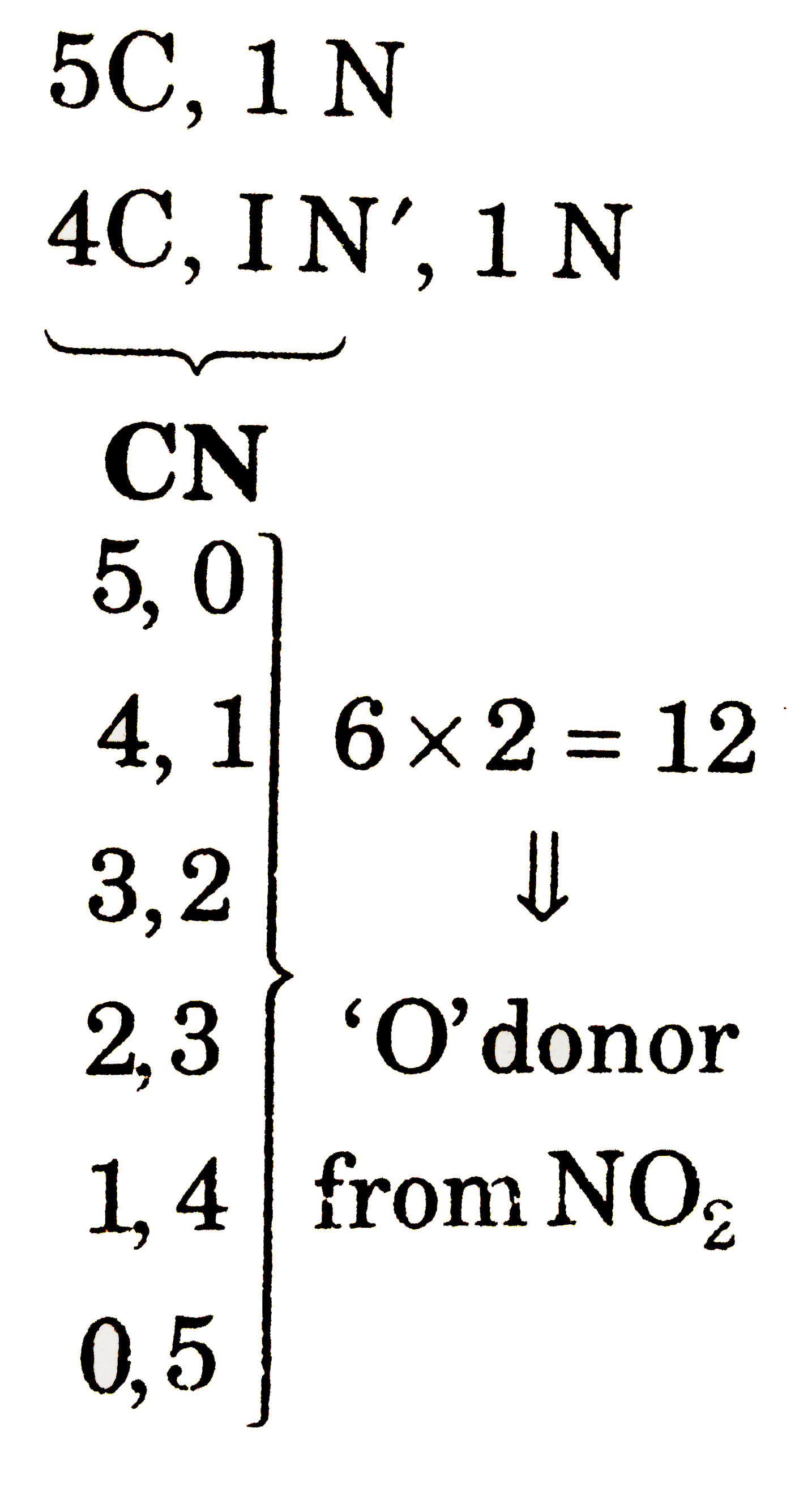
|
|
| 1605. |
Write the reactions of carboxylic acid with the following reagents. (Write the chemical equations) (i) Thionyl chlorides (SOCl_2) |
|
Answer» |
|
| 1606. |
Which of the following species would be expected as paramagnetic ions |
|
Answer» `CU^(+)` |
|
| 1607. |
What is a complex ion ? |
| Answer» Solution :A COMPLEX ion is a charged particle in which a central METAL ion is surrounded by a definite NUMBER of molecules or IONS CALLED ligands. | |
| 1608. |
What is the role of Bithional in toilet soaps ? |
| Answer» SOLUTION :To IMPART ANTISEPTIC PROPERTIES to SOAPS. | |
| 1609. |
Writeshortnotes on (i) Mustard oil reactions (ii) Carbylaminereaction (iii) Gabrielpathalimidesynthe$is |
|
Answer» Solution :Mustard oil reaction :(a) When primary amines aretretedwith carbon disulphide` (CS_(2))` , N - alkyldithiocarbonic acid is formedwhichon subsequent treatment with ` HgCI_(2)` givesan alkyl isothiocyanate . ` (##SUR_CHE_XII_V02_QP_E01_040_S01.png" width="80%"> (b)whenaniline is treatedwith carbondisuphide , or heatedtogether , S-diphenylthio urea is formed, whichon boiling with strongHCI, Phenyl isothiocyanate (phenyl mustedoil) , is formed Thesereactions are knowsas Hofmann-Mustard oil reaction.This testis usedto identifythe primaryamines. (ii) Carbylaminereaction : Aliphatic (or) aromaticprimaryaminesreact WITHCHLOROFORM and alcpholicKOH to giveisocyanides(carbylamins) , whichhas an unpleasantsmell. This reactionis known as carbylamines TEST. This testused to identifythe primaryamines ` underset(" Ethylamine")(C_(2)H_(5) - NH_(2)) + underset("Chloroform")( CHCI_(3) + 3KOH) to underset(" Ethylisocyanide")(C_(2)H_(5)- NC + 3KCI + 3H_(2)O)` (iii) Garbriel phthalimidesynthesis : Gabriel synthesis is used for thepreparation of Aliphaticprimaryamines. Phthalimide on treatment with ethanolicKOHforms potassiumsalt of Phthalimidewhichonheatingwith alkylhalidefollowedbyalkaline hydrolysis givesprimary AMINE. Aniline cannotbe preparedby thismethodbecausethe arylhalides do notundergo nucleophilicsubstitution with the anionformedby phthalimide. 
|
|
| 1610. |
White phosphorus on reaction with lime water gives calcium salt of an acid(A) along with a gas (X). Which of the following statement is correct for gas (X) ? (I) (A) on heating gives gas (X). (II) (X) is more basic than ammonia. (III) (X) is a poisonous gas and possess smell of rotten fish. (IV) The solution of (X) in water decomposes in presence of light giving red phosphorus and H_(2) Correct code is : |
|
Answer» I,II,III,IV |
|
| 1611. |
Which of the following is an example of co-polymer ? |
| Answer» Answer :D | |
| 1612. |
Which of the following is state function (a)q+w,(b)q(c )w(d) heating isobaric process (e) work in adiabatic process :- |
|
Answer» a,B,c |
|
| 1613. |
Which inorganic precipitate acts as a semipermeable membrane ? |
|
Answer» CALCIUM phosphate |
|
| 1614. |
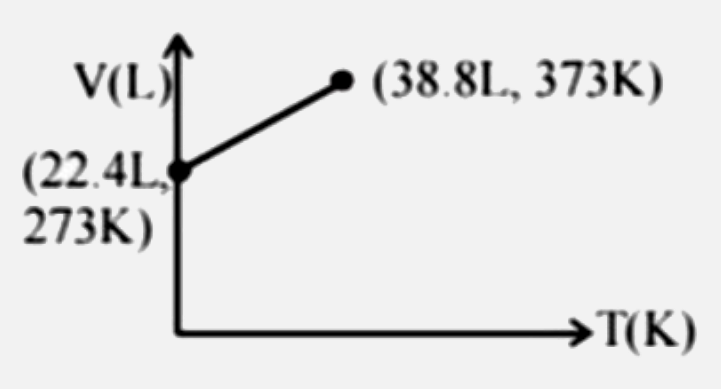
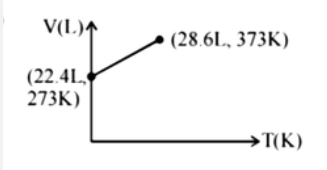
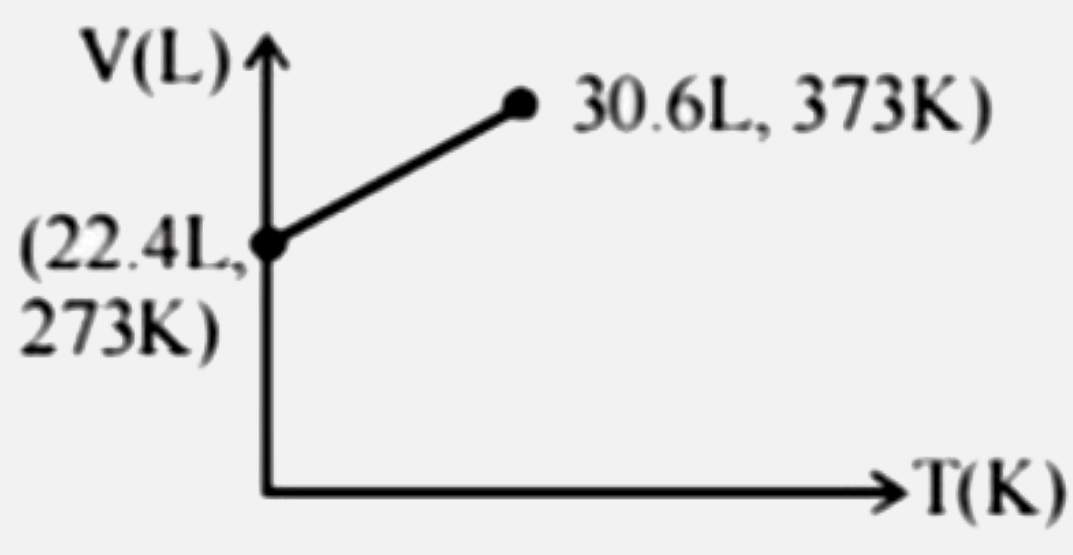
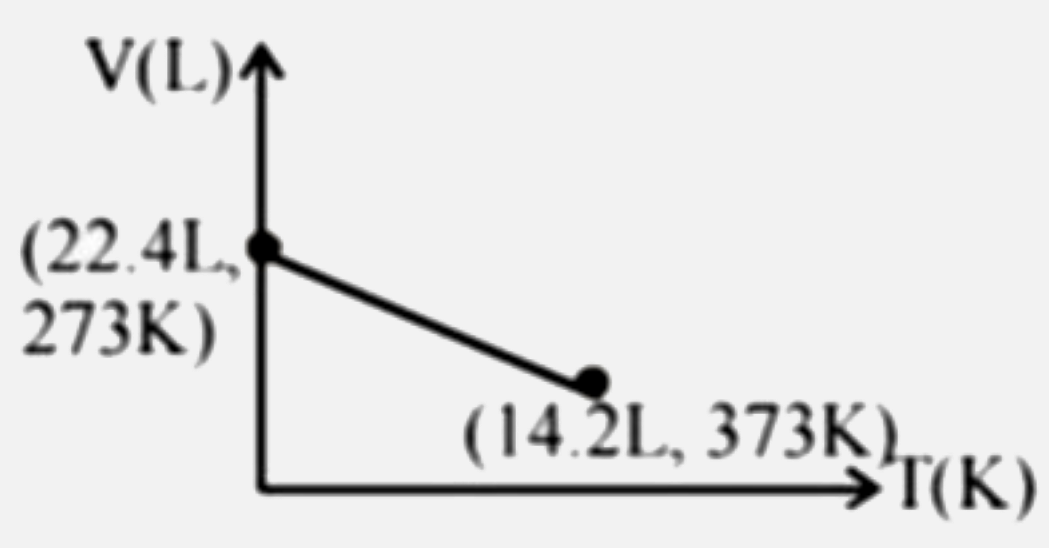
Which of the following volume-temperature (V-I) plots represents the behaviour of 1 "mole" of an ideal gas at the atmospheric pressure? |
|
Answer»
|
|
| 1615. |
What is enthalpy of atomisation ? Explain |
|
Answer» Solution :The energy required to form a 1 mole of gaseous ATOMS from its elements in its standard state is called standard enthalpy of atomisation. The transition elements have high enthalpies of atomisation because of presence of strong bonds between the atoms. The enthalpy of atomisation increases with the increase in number of unpaired electrons which results in formation of strong bonds DUE to strong interatomic interactions. The elements of 4d adn 5d series have greater enthalpy of atomisation than CORRESPONDING elements of first transition series which results in much more metal-metal bonding in compounds of the heavy transition elements.  In first transition series, Zn has MINIMUM enthalpy atomisation because of ABSENCE of unpaired electrons |
|
| 1616. |
Which one of the followingcompound is foundabuandantly in nature ? |
|
Answer» FRUCTOSE |
|
| 1617. |
What happens when sodium azide undergoes thermal decomposition? |
| Answer» SOLUTION :PURE nitrogen gas can be OBTAINED by the thermal decomposition of sodium azide about 575 K `2NaN_(3)OVERSET(575K)rarr 2Na+3N_(2)` | |
| 1619. |
When a certain conductance cell was filled with 0.1 mol L^(-1)KCl, it has a resistance of 85Omegaat 25^(@)C. When the same cell was filled with an aqueous solution of 0.052 mol L^(-1) of an electrolyte solution, the resistance was 96 Omega . Calculate the molar conductivity of the electrolyte at this concentration (Conductivity of 0.1 mol L^(-1) KCl solution is 1.29 xx 10^(-2) S cm^(-)) |
|
Answer» where G is conductance and `G^(**)` is CELL constant `G^(**) = (1.29 xx 10^(-2) S cm^(-1))/(1//85) = 1.0965 cm^(-1)` `kappa` for `0.052 mol L^(-1)` solution . `kappa = G xx G^(**) = (1)/(96) xx 1.0965= 1.142 xx 10^(-2) S cm^(-1)` `Lambda = (kappa xx 1000)/(M)` `= (1.142 xx 10^(-2) xx 1000)/(0.052)` `= 219.6 S cm^(2) mol^(-1)` |
|
| 1620. |
Which gas is obtained on dissolving zinc is not dilute nitric acid? |
|
Answer» `NH_3` |
|
| 1621. |
When one mole of an ideal gas is compressed to half of its initial volume and simultaneously heated to twice its initial temperature , the change in entropy of gas (DeltaS) is : |
|
Answer» `C_(p.m) In 2` |
|
| 1622. |
x= number of compound which undergoes Tautomerisation ot from an Aromatic product. |
Answer» 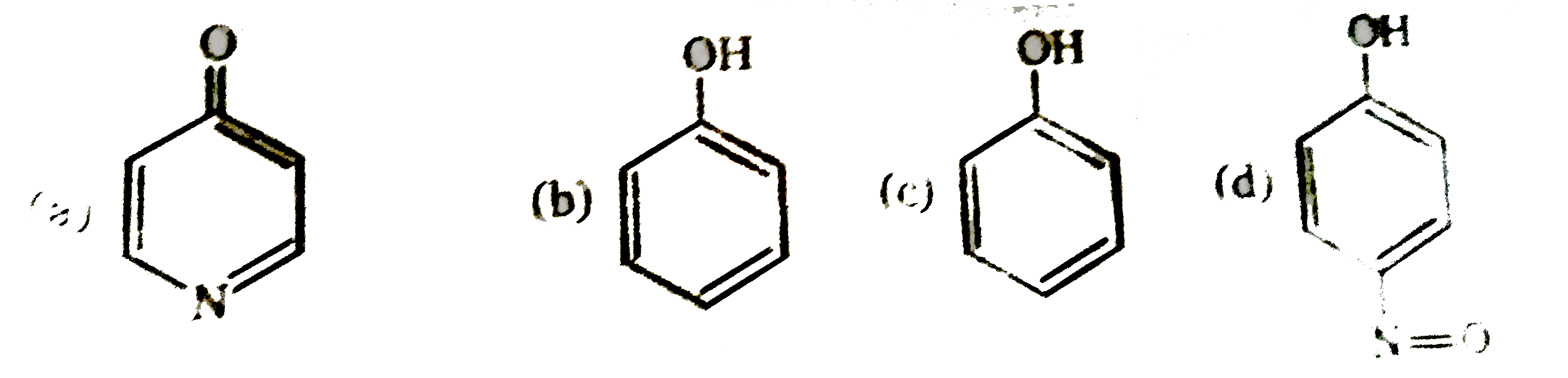
|
|
| 1623. |
When Manucame fromschool,he sawhismothertalkingtoaperson who claimedthat hewouldpolish hertarnishedsilver/goldbangleschargingonlyanominalamountof money. Outof curiosity, Manuaskedthepersonconcernedas to how wouldhepolishthebangles ? Themanshowedhimasliveryliquid.Usinghis knowledgeofchemistry,Manuimmediately sawthroughthe trickandaskedhis mothernotthegetthebangles polishedandrequested the mantopleasegoaway.Read thispassage and answerthefollowing questions :(i) Namethe silveryliquidwithwiththe banglesweretobe rubbed.(ii) Why did Manuaskhismothernotto getthebanglespolished ? (iii) Whatbenefitdoes the manderivefromrubbingthesilver /goldbangleswithsilvery liquid ?(iv) Asastudentofchemistry, whatinitiativewouldyou take to savetheinnocentwomenin thestreetsandmohallasfrombeingcheatedbyquacks ? |
|
Answer» Solution :(i) Thesilveryliquidismercury. (ii) Whensilver /goldisrubbedwithmercury, itformsas amalgamwhichdissolvesin theliquidmercury.Due toremovalofthetarnishedsurface ofthe silver /GOLD,banglesgive a shininglookbuttheweightof `2Ag +x Hg tounderset ("Silver AMALGAM") (Ag_2 Hg_x), 2 Au +xHg tounderset ("Gold amalgam")(Au_2Hg_x) ` thebanglesdecreasesduetolossof somesilver /golddue toamalgamformation. That iswhyManu ASKED his mothernottogetthebanglespolished. (iii) Themanisbenefited byrecoveringsilver /gold behind. (IV) A SENSEOF awareness shouldbecreated inthe mindsof innocentwomen byshowingskirts, plays,dramas andprogrammeson TV thatpolishingof silver/goldbanglesandotherornamentsshouldbegot done fromreputed jewelleryshopsin thetownand notfromirresponsibleandunauthorisedpersons.Membersof socialorganisationscanalso undertakedoortocontractprogrammestodecouragewomenfrom gettingsuch servicesfromunknownpersons. |
|
| 1624. |
Which is the best definition of ''heat of neutralization'' |
|
Answer» The heat set free when one gram molecule of a base is neutralized by one gram molecule of an acid in dilute SOLUTION at a stated TEMPERATURE |
|
| 1626. |
When 0.01 moles of sodium hydroxide are added to a buffer solution, its pH changes from 4.745 to 4.815. The buffer capacity of the buffer solution is : |
|
Answer» `0.07` |
|
| 1627. |
Which one of the following transition metal ions shows magnetic moment of 5.92 BM? |
|
Answer» `Mn^(2+)` `=sqrt(n(n+2))=5.92` i.e., n=5 Number of unpaired electrons in `Mn^(2+)=5` Number of unpaired electrons in `Ti^(3+)=1` Number of unpaired electrons in `Cr^(3+)=3` Number of unpaired electrons in `Cu^(2+)=1` Number of unpaired electrons in `CO^(2+)=3` THUS `Mn^(2+)` have magnetic moment`=5.92BM` |
|
| 1628. |
Which of the following reactants on reaction with conc. NaOH followed by acidification gives the following lactone as the only product ? |
|
Answer»
|
|
| 1629. |
When acetamide reacts with Br_2 and caustic soda, then we get |
|
Answer» ACETIC acid |
|
| 1630. |
Whattypeof bondinghelp in stabilisingthe alpha - helix structureof proteins ? |
| Answer» Solution :HYDROGEN bondingpresentbetween - NH GROUPOF eachamino acid residueand the `GT C = O`of anadjacentof the helixstructureof PROTEINS . | |
| 1631. |
Which of the following represents the correct IUPAC name for the compounds concerned? (1) 2,2 - dimethylpentane or 2 - demethylpentane (2) 2,4,7 - trimethyloctane or 2,5,7 - trimethyloctane (3) 2 - chloro - 4 - methylpentane or 4 - chloro - 2 - methyl - pentane (4) But - 3 - yne - 1 - ol - 1 - yne. |
|
Answer» Solution :(1) 2,2 - dimethylpentane (two alkyl groups are on the same carbon and hence the locant is REPEATED twice). (2) 2,4,7 - trimethyloctane (since 2,4,7 locant set is lower than the set 2,5,7). (3) 2 - chloro - 4 - methylpentane (alphabetical ORDER of SUBSTITUENTS is MAINTAINED). (4) But - 3 - yne - 1 - ol (using lower locant for the PRINCIPAL functional group). |
|
| 1632. |
Whichbiomolecule is themostabunadant in all livingorganisms ? |
|
Answer» Carbohydrates |
|
| 1633. |
What is the position of Aluminium metal among the metals obtained from the earth crust? |
| Answer» ANSWER :B | |
| 1634. |
Which hydride is an ionic hydride ? |
|
Answer» `NH_3` |
|
| 1635. |
What quantity of electricity is required for complete reduction of all Ag^(+) from 1.0 M AgNO_(3) is 250 ml aqueous solution ? |
|
Answer» 2412.5 C 1 MOLE of `Ag^(+) =96500C (1F)` |
|
| 1636. |
Which one of the following is not a cross linked polymer? |
|
Answer» POLY PROPYLENE |
|
| 1637. |
When an acidified solution of ferrous ammonium sulphate is treated with KMnO_4 solution, the ion which is oxidised is: |
|
Answer» `FE^(2+)` |
|
| 1638. |
The vapour pressure of acetone at 20^(@)C is 185 torr. When 1.2 g of a non-volatile substance was dissolved in 100 g of acetone at 20^(@)C , its vapour pressure was 183 torr. The molar mass ( " g mol"^(-1)) of the substance is : |
|
Answer» 32 `=185` torr Vap. pressure of solution, `p=183` torr MOLAR mass of SOLVENT, `M_(A)=58 "g mol"^(-1)`, `w_(B)=1.2 g, w_(A)=100 g` `(p_(A)^(@)-p)/(p_(A)^(@))=x_(B)=(n_(B))/(n_(A))` `(p_(A)^(@)-p)/(p_(A)^(@))=(w_(B))/(M_(B))xx(M_(A))/(w_(A))` `(185-183)/(185)=(1.2)/(M_(B))xx(58)/(100)` `(2)/(185)=(1.2xx58)/(M_(B)xx100)` `:.M_(B)=(1.2xx58xx185)/(2xx100)=64.38` |
|
| 1639. |
Which of the following complexes can exists as enantiomers ? Draw their structures (a) cis-[Co(NH_(3))_(4)Br_(2)]^(+) "" (b) cis-[Cr(H_(2)O)_(2)(en)_(2)]^(3+)"" (c )[Cr(gly)_(3)] (d) [Cr(en)_(3)]^(3+) "" ( e) cis-[Co(NH_(3))Cl(en)_(2)]^(2+) "" (f) trans-[Co(NH_(3))_(2)(en)_(2)]^(2+) |
|
Answer» |
|
| 1640. |
Write the structure of following compound: 1-Bromo-4-sec-butyl-2-methylbenzene |
Answer» SOLUTION :The STRUCTURE of 1-Bromo-4-sec-butyl-2-methylbenzene is GIVEN below: 
|
|
| 1641. |
Which of the following is water soluble . |
|
Answer» `Roverset(+)NH_(3)Xbar` |
|
| 1642. |
There are several criteria of purity of organic compounds. Which is considered to be the best: |
|
Answer» MELTING point |
|
| 1643. |
When This sulphate ion is oxidized by iodinc, new product 'X' is formed. The number of s-s linkage isi/are present in 'X'? |
|
Answer» (`beta` -form and `omega` -form)  Cyclic TRIMER (`alpha`-form), 3 S-O-S bonds, 12 S - O bonds Cyclic TRIMER (`alpha`-form), 3 S-O-S bonds, 12 S - O bonds  Infinite helical chain (`beta` -form), each of the tetrahedrom is aboe to share two oxygens |
|
| 1644. |
Which is not a disproportionation reaction ? |
|
Answer» Since same ATOM is not reduced & oxidised. |
|
| 1645. |
Which of the following has highest melting point ? |
|
Answer» n-pentane |
|
| 1646. |
Which of the following staments is incorrect? |
|
Answer» Disinfectants are chemical substance which helps to kill the micro-organisms. |
|
| 1647. |
Which of the following pairs has bleaching property |
|
Answer» `O_(3)` and `NO_(2)` |
|
| 1648. |
Which type of complex is cryolite ? |
| Answer» SOLUTION :CRYOLITE, `Na_3[AlF_6]` is anionic complex. This is because the complex ION `[AlF_6]^(3-)` is an ANION. | |
| 1649. |
When H_(2)O_(2) react with N_(2)H_(4) then H_(2)O_(2) oxidised N_(2)H_(4). The no. of mole of H_(2)O_(2) required to oxidised 64gm N_(2)H_(4). |
|
Answer» `N_(2)H_(4)+2[O]rarr N_(2)UARR +2H_(2)O` `UL(BAR(N_(2)H_(4)+2H_(2)O rarrN_(2)uarr+4H_(2)O))` |
|
| 1650. |
Which is the product when sodium phenoxide is heated with ethyl Iodied ? |
|
Answer» thoxy BENZENE 
|
|