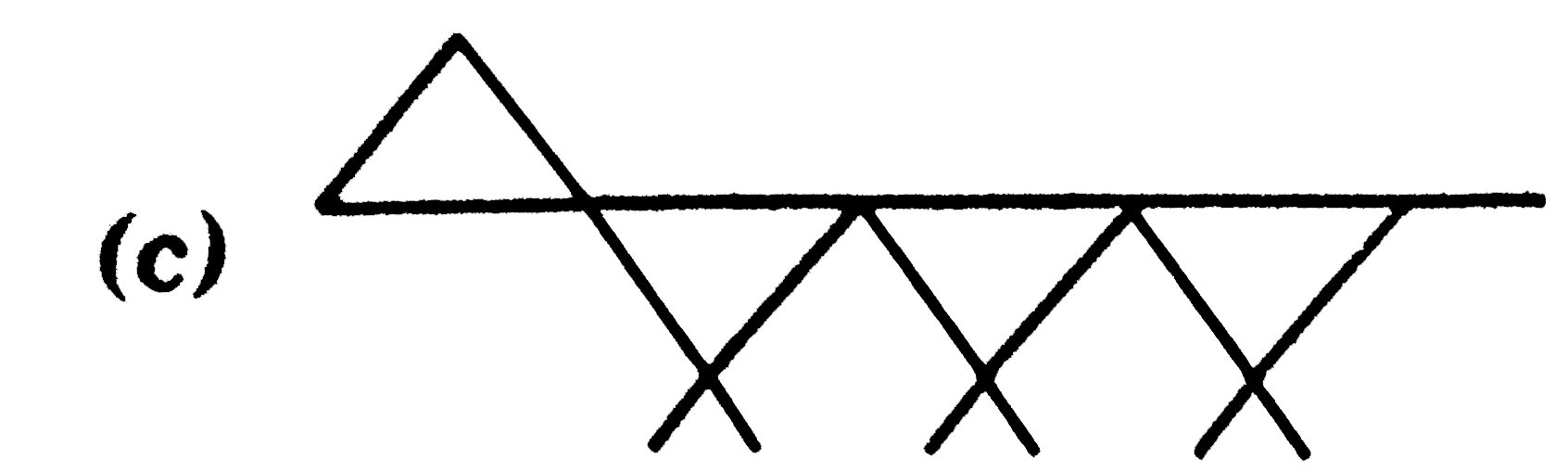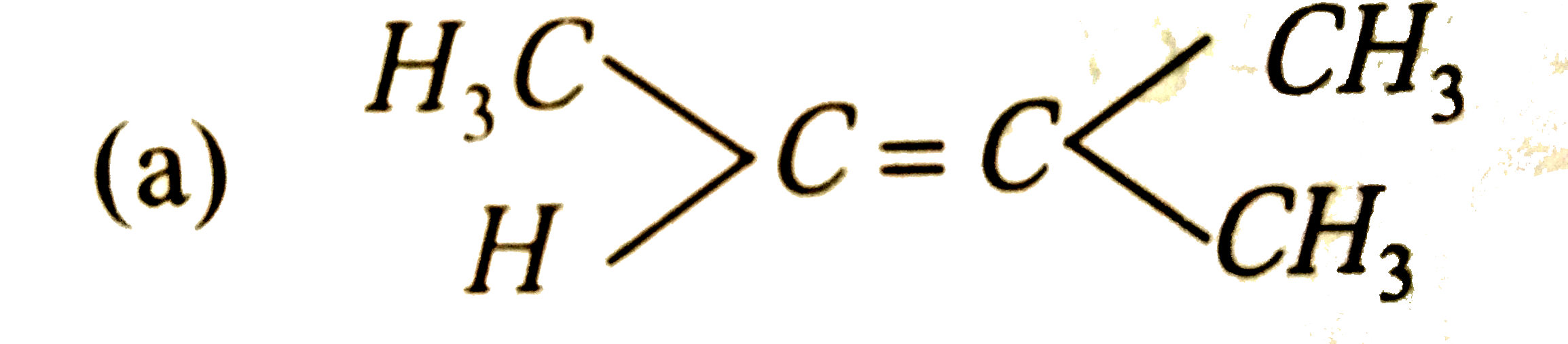Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 1551. |
The solubility of Agcl (K_sp=1.2xx10^(-10)) in a 0.10 M NaCl solution is : |
|
Answer» 0.1 M |
|
| 1552. |
Which of the following is the least reactive towards nucleophile? |
|
Answer» `{:(CH_3CH_2CH(CH_3)CH_2NH_2),(""darrNH_3),(CH_3CH_2CH(CH_3)CH_2Br):}` |
|
| 1553. |
Water and hydrogen peroxide illustrate the law of: |
|
Answer» RECIPROCAL proportion |
|
| 1554. |
Which of the following metals will give H_2 on reaction with NaOH. |
| Answer» Answer :A | |
| 1555. |
Which one among the following non-metals liquid at 25^@C |
|
Answer» Bromine |
|
| 1556. |
Which of the following potassium compounds is known as "Pearl ash" |
| Answer» Answer :C | |
| 1557. |
Which of the following is not Lanthanides? |
|
Answer» CURIUM |
|
| 1558. |
What amount of dioxygen (in gram) contains 1.8xx10^(22) molecules? |
|
Answer» 9.6 |
|
| 1559. |
The reduction of nitrobenzene with Zn and KOH (aq.) |
|
Answer» Benzene |
|
| 1560. |
Which two of the following salts are used for preparing iodized salt? (i) KIO_(3), (ii) KI (iii) I_(2) (iv) HI |
|
Answer» (i) and (ii) |
|
| 1561. |
What is determined by Ultra-centrifuge technique in polymers ? |
|
Answer» SOLUTION |
|
| 1562. |
Which one of the following methods is neither meant for the synthesis nor for separation of amfoes? |
|
Answer» Hinsberg method |
|
| 1563. |
Which of the following statements regarding DNA fingerprinting is incorrect? |
|
Answer» It is used in forensic LABORATORIES for identification of criminals. |
|
| 1564. |
What is the change in internal energy when a gas contracts from 377 ml to 177 ml under a constant pressure of 1520 torr, while at the same time being cooled by removing 124 J heat ? [Take : (1 L atm) = 100 J] |
| Answer» ANSWER :B | |
| 1565. |
Which of the following methods will give allyl chloride? |
|
Answer» Reaction of propane with HCl |
|
| 1566. |
What is the value of i when solute is associated and dissociated ? |
| Answer» SOLUTION :`ilt 1` when SOLUTE is associated and `igt 1` when solute is dissociated . | |
| 1567. |
What is the number of possible isomers for the octahedral complex [Co(NH_(3))_(2)(C_(2)O_(4))_(2)] ? |
|
Answer» 1 |
|
| 1568. |
When KOH solution is added to potassium dichromate solution,the colour of solution changes to yellow because : |
|
Answer» chromate ION changes to dichromate ion |
|
| 1569. |
Two isomeric mononitro derivaters (B) and (C) are obtained by the nirtration of an organic compound (A), C_(7) H_(8)O. Treatment of (A0 with acetyl chloride products (D) which on reaction with CrO_(2)Cl_(2) gives (E) whose oxidation with neutralKMnO_(4) followed by acidification gives (F). Compound (F) on heating gives pheonl. (A) on treatmentwith alkaline C_(6) H_(5)SO_(2)Cl produces. (A) on treatmentwith alkaline C_(6) H_(5) SO_(2)Cl produces (G) which on oxidiation with KMnO_(4)gives (H). Hydrolysis of (H) also gives (F). Give teh structures of (A) to (H). |
Answer» SOLUTION :The REACTION SUGGEST that `(A)` is 
|
|
| 1570. |
Zeta potential (or electrokinetic potential) is the a. Potential required to bring about coagulation of a colloidal sol. b. Potential required to give the particles a speed of 1cms^(-1) in the sol. |
| Answer» Solution :The potential DIFFERENCE that EXISTS between the stationary layer of compensating charges and the diffuse layer (present is the body of the solution) is CALLED zeta potential or electrokinetic potential. | |
| 1571. |
Which of the following statement is incorrect about the equation? C_(2)H_(5)OH(l)+3O_(2)(g) to 2CO_(2)(g)+3H_(2)O(l),Delta_(r)H^(-)=-1367" kJ "mol^(-1) |
|
Answer» the reaction is endothermic in nature. `Delta_(r)H=-1367" kJ "mol^(-1)` The above equation describes the combustion of liquid ethanol at constant temperature and pressure. The negative sign of enthalpy change INDICATES that this is an exothermic reaction. |
|
| 1572. |
Which of the following is amphoteric oxide ? Mn_(2)O_(7),CrO_(3), Cr_(2)O_(3), CrO, V_(2)O_(3), V_(2)O_(4) |
|
Answer» `V_(2)O_(5), Cr_(2)O_(3)` |
|
| 1573. |
The unit of cell constant of a conductivity cell is _____________. |
| Answer» SOLUTION :`CM^(-1)` | |
| 1574. |
When a system is at equilibrium. |
|
Answer» 1. The concentration of reactants and products BECOMES equal |
|
| 1575. |
Which of the following is not applicable to Nylon-6, 6 ? |
| Answer» Answer :D | |
| 1576. |
Which of the following statements are applicable to a balanced chemical equation of an wlwmwntry reaction ? |
|
Answer» ORDER is same as molecularicty. |
|
| 1577. |
What istheroleofastabilizerin frothfloatationprocess? |
| Answer» SOLUTION :CHEMICAL compoundslikecresols and anilinewhichtendtostabilizethefrotharecalledfrothstabilizers. | |
| 1578. |
which of the following acid will be formed only when P_2O_3? |
|
Answer» `HPO_3` |
|
| 1579. |
The sweeteners value of aspartame in comparison to canesugar is |
|
Answer» 550 |
|
| 1580. |
Which of the following belong to the class of natural polymers: |
|
Answer» Proteins |
|
| 1581. |
Which of the following statements is not correct :- |
|
Answer» Oxidising power `=SiCl_(4) lt SnCl_(4) lt PbCl_(4)` (ii) size of halogen `(uarr)` = polarisiation `(uarr)` = ionic character `(darr)` (iv) Liquification of nobel gases increases down the group, due to increasing in London force. |
|
| 1582. |
Which among the following metals crystallise as a simple cube ? |
|
Answer» Polonium |
|
| 1583. |
The specific gravity of H_(2)SO_(4) after complete discharging becomes |
|
Answer» 1.17 |
|
| 1584. |
Which compound shows S_(N)1 mechanism as well as optical isomerism ? |
|
Answer» BENZYL CHLORIDE |
|
| 1585. |
Which of the following reacts with nitrous acid |
|
Answer» Acetamide `underset(2^(@)"Nitroalkane")overset(C_(2)H_(5))overset(||)(CH_(3)CHNO_(2))overset(HONO)rarrunderset("Pseudonitrol(blue)")overset(N=O)overset(|)((CH_(3))_(2)CNO_(2))` |
|
| 1586. |
What volume of 0.8 M solution contains 0.1 mole of solute |
|
Answer» 500 ml |
|
| 1587. |
Which of the following is not correct in case of boron nitride ? |
|
Answer» It is also called borazon |
|
| 1588. |
Which diagram is correct for (9)/(4) oxygen shared per tetrahedra? |
|
Answer»
|
|
| 1589. |
When primary bromides are treated with a solution of Nal in pure acetone preciptate generates within 3 minutes at 25^(@)C whereas primarychlorides when treated with same reagent precipitate generates within 6 minutes at 50^(@)C. Explain. |
|
Answer» Solution :The reaction is probably of the `S_(N)2` type involving a bimolecular attack of the iodide ion upon the carbon atom CARRYING the chloride or BROMINE. Now `BR^(-)` is better leaving group when `Cl^(-)` (as it is a weaker base). So the rate of the reaction for bromides is faster. `{:(RBr+Nal to Rl+NaBr downarrow),(RCl+Nal to Rl +NACL downarrow):}`. |
|
| 1590. |
Use of chloropicrin is as…………….. |
|
Answer» explosive |
|
| 1591. |
Write rate law and order of the following reaction :AB+C_2 to AB_2C+C (slow) AB_2+C to AB_2C (fast) |
| Answer» SOLUTION :RATE =`K[AB][C_2]`, ORDER =1+1=2 | |
| 1592. |
Total number of optical active stereoisomers of the following compound is : CH_(3)-CH=CH-CHCI-CH=C=CH-CH=CH-CH_(3) |
|
Answer» 8 |
|
| 1593. |
What type of isomers are the following [Co("en")_(3)]Cr(CN)_(6)]and[Cr("en")_(3)][Co(CN)_(6)] |
|
Answer» Co-ordination |
|
| 1594. |
Which of the following compounds is used as refrigerant ? |
|
Answer» CARBON tetrachloride |
|
| 1595. |
What is the role of adding an electrolyte in peptisation ? |
| Answer» Solution : IONS of ONE type are adsorbed on the precipitates. On shaking the precipitates are converted into COLLOIDS DUE to repulsion between the particles carrying the same charge. | |
| 1596. |
Write the IUPAC name of CH_(3)-C-=C-CH_(2)-overset(O)overset(||)(C)-OH |
| Answer» SOLUTION :Pentan-3-yne-oic ACID. | |
| 1597. |
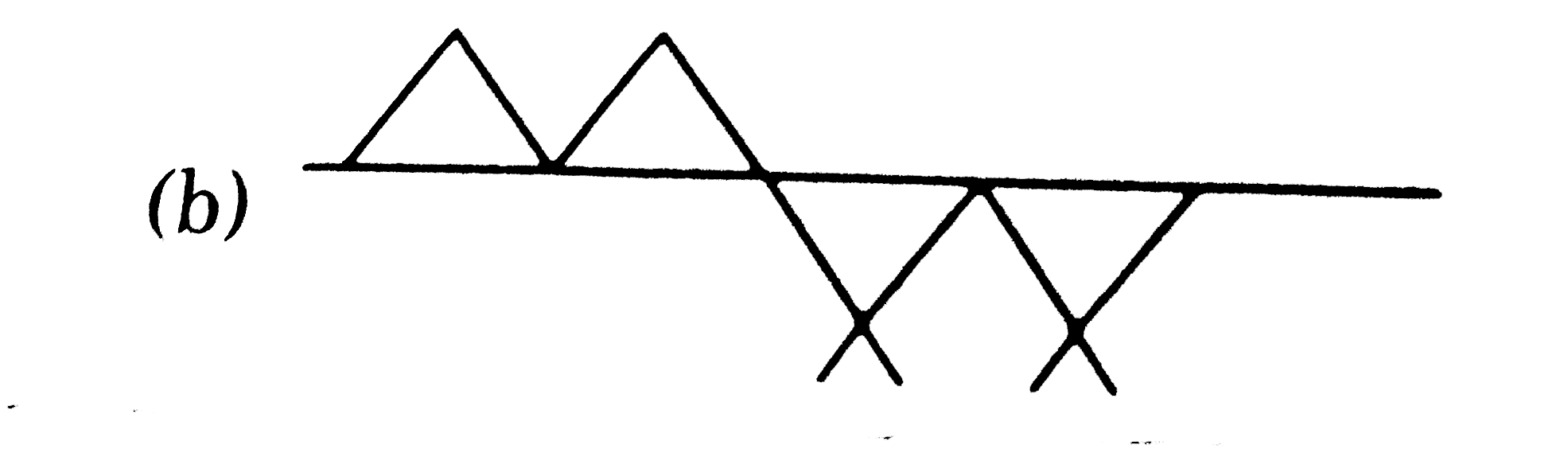
Which of the following hydrocarbons has the lowest dipole moment |
|
Answer»
|
|
| 1598. |
Using the calculated emf value of zinc and copper electrode, calculate the emf of the following cell at 25^(@)C. Zn(s)abs(Zn^(2+)(aq, 1M))abs(Cu^(2+)(aq, 1M))Cu(s) |
|
Answer» Solution :`Zn_((s))abs(Zn_(AQ, 1M)^(2+))abs(Cu_(aq, 1M)^(2+))Cu_((s))` `E_(Zn//Zn^(2+))^(@)=0.76 V` `E_(CU^(2+)|Cu)^(@)=0.34V` `E_("Cell")^(@)=E_("ox")^(@)+E_("red")^(@)` `""=0.76+0.34` `E_("Cell")^(@)=1.10V` |
|
| 1599. |
Time required to decompose SO_(2)Cl_(2) to half of its initial amount is 60 minutes. IF the decomposition is a first order reaction. Calculate the rate constant of the reaction. |
| Answer» SOLUTION :`0.01155 MIN^(-3)` | |
| 1600. |
Which of the following is the correct order of flocculating for the coagulation of positive ion ? |
|
Answer» `[FE(CN)_(6)]^(4-) GT SO_(4)^(2-) gt Cl^(-) gt PO_(4)^(3-)` |
|