Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 92651. |
2 moles of an ideal gas initially present in a piston fitted cylinder at 300 K, and 10 atm are allowed to expand against 1 atm but the piston was stopped before it stablished the mechanical equilibrium. If temperature were maintained constantthrough out the change and system delivers 748.26 J of work, determine the final gas pressure and describe the process on PV diagram. |
|
Answer» SOLUTION :`W_("irrv")=-748.26` `W_("irr")=-P_("EXT")[1//P_(2)-1//P_(1)]nRT` `P_(2)` = 4 ATM |
|
| 92652. |
Two moles of ammonia were found to occupy a volume of 5 L at 27^(@)C. Calculate the pressure using van der Waals equation (a="4.17 bar L"^(2)"mol"^(-2), b="0.0371 L mol"^(-1)). |
|
Answer» Solution :n= 2 V = 5 LITRES `T = 273 + 27 = 300 K` `a = 41.7 "atm lit"^(2) "mole"^(-2)` `b = 0.0371 "lit mole"^(-1)` `R = 0.082 "lit atm deg"^(-1) "mole"^(-1)` Applying van der Waals equation for n moles `(p + (a n^(2))/(V^(2))) (V-n b) = nRT` `(p+(4.17 xx 2^(2))/(5^(2))) (5-2 xx 0.0371) = 2 xx 0.082 xx 300` p = 9.33 atm. |
|
| 92653. |
2 moles of acetaldehyde are warmed in the presence of 10% Ba(OH)_2 to give a product which dehydrates to a compound X. The compound on reduction with NaBH_4 will give |
|
Answer» `CH_(3)CH=CHCHO` `CH_(3)-overset(O)overset(||)underset(H)underset(|)(C)+overset(H)overset(|)(C)H_(2)-overset(O)overset(||)(C)-H overset(BA(OH)_(2))toCH_(3)-overset(OH)overset(|)underset(H)underset(|)(C)-overset(H)overset(|)(C)H-overset(O)overset(||)(C)-H overset(DELTA)underset(-H_(2)O)to CH_(3)-CH=CH-overset(O)overset(||)(C)-H overset(NaBH)underset({:("Selective"),("reduction"):})toCH_(3)-CH=CH-CH_(2)OH` |
|
| 92654. |
2 moles monoatomic ideal gas is expanded isothermally and reversibly from 5 L to 20 L at 27°C. deltaH, deltaU, q and w for the process respectively are (log 2 = 0.3) |
|
Answer» ZERO, Zero, Zero and Zero |
|
| 92655. |
2 moles carbon and 1.5 moles of oxygen gas are reacted in a container to produced CO and CO_(2) or both such that none of reactant is left. Find moles of CO, CO_(2) produced. |
|
Answer» `CO=1"MOLE",CO^(2)=1"mole"` |
|
| 92656. |
2 MOLE of PCl_5 were heated in a closed vessel of 2 litre capacity. AT equilibrium 40% Of PCl_5 dissociated into PCl_3and Cl_2.Find the value of equilibrium constant. |
|
Answer» 0.267 |
|
| 92657. |
2 mole of ideal momoatmic gas was subjected toreversibleadiabaticcompression from initianl state of P= 1 andT = 300 Ktill the pressrue is 4sqrt(2) atm and temperature is TK . The gas is then subjected to reversible isothermal expansion atT K tilltheinternalpressureis one atm . The gas is nowisobarically cooled to attaininitialstate. Find W_(Net) ( in calorie) forwhole process . [Use In2=0.7] |
|
Answer» ` = 600 K` `W_(I)= W_("rev")= nC_(V)[T_(2)- T_(1)] = 2 xx (3)/(2) R xx 300` `= 900 R` `W_(II)= - 2.303 nRT LOG .(P_(1))/(P_(2)) = - 2xx R xx 600 In ((4sqrt(2))/(1))` `W_(III) = - PDeltaV = - nRT = - 1200R xx (5)/(2) xx 0.7 = - 2100 R` `W_("Total") = 900 R - 2100 R + 600 R` ` = - 600 R = - 1200 CAL` |
|
| 92658. |
2mole of Fe_(2)C_(2)O_(4) is oxidized by x mole of Cr_(2)O_(7)^(2-)in acidic medium , x is |
|
Answer» |
|
| 92659. |
2 mole of ethanol is dissolved in 8 mole of water. The mole fraction of water in the solution is |
|
Answer» 0.2 `:. "MOLE fraction of water"=("Moles of water")/("Total moles")` `=8/10=0.8` |
|
| 92660. |
2 mole of ethanol are burnt. The amount of CO_2 obtained will be: |
|
Answer» 132 g |
|
| 92662. |
2 mol of N_(2) is mixed with 6 mol of H_(2) in a closed vessel of one litre capacity. If 50% of N_(2) is converted into NH_(3) at equilibrium, the value of K_(c) for the reaction N_(2(g))+3H_(2(g))iff2NH_(3(g)) is |
|
Answer» `4//27` `K_(C)=((2)^(2))/(1xx(3)^(3))=(4)/(27)` |
|
| 92663. |
2 mol of N_(2) is mixed with 6 mol of H_(2) in a closed vessel of one litre capacity. If 50% of N_(2) is converted into NH_(3) at equlibrium, the value of K_(c) for the reaction N_(2(g))+3H_(2(g))hArr2NH_(3(g)) is |
|
Answer» `4//27` `50%` Dissociation of `N_(2)` TAKE PLACE so, At equlibrium `(2xx10)/(100)=1,` value of x=1 `K_(C)=([2]^(2))/([1][3]^(3))=4/27so, K_(c)=4/27` |
|
| 92664. |
2 mol of an ideal gas expands isothermally and reversibly from 1 litre to 10 litres at 300 K. What is the enthalpy change? |
|
Answer» 4.98 KJ |
|
| 92665. |
2-Methylpropene upon hydroboration-oxidation gives |
|
Answer» 2-Methyl-1 propanol |
|
| 92666. |
2-Methylpropane on monochlorination under photochemical conditions give |
|
Answer» 2-chloro-2-methylpropane as major PRODUCT RATIO of (A): (B) is 5:9 |
|
| 92667. |
2-Methylpropane onmonochlorination under photochemical condition give |
|
Answer» 2-chloro-2-methylpropane as MAJOR PRODUCT |
|
| 92668. |
2-Methylpropan-2-ol on oxidation underdrastic condition gives _________ as the final product. |
|
Answer» methanoic acid |
|
| 92669. |
2-methylpropan-2-ene overset(H_2SO_4//H_2O) |
| Answer» Solution :`UNDERSET(("2 - methyl propan - 2- ENE"))(CH_3-underset(CH_3)underset(|)C=CH_2)OVERSET(H_2SO_4//H_2O)to underset(("2-methyl PROPANE - 2-ol"))(CH_3 -underset(CH_3)underset(|)overset(OH)overset(|)C-CH_3)` | |
| 92671. |
2-methylpropan -1-ene overset(H_(2)SO_(4)//H_(2)O) to ? |
|
Answer» Solution :`underset("2-methyl propan-1-ene") (CH_(3)-underset(CH_(3)) underset(|)C= CH_(2)+H_(2)O underset(H_(2)SO_(4)) to CH_(3) -underset(CH_(3)) underset(|)OVERSET(OH) overset(|)C-CH_(3)` It follows Markownikoff's addition. |
|
| 92672. |
2-Methylpenta-2,3-diene is achiral because it has: |
|
Answer» a PLANE of SYMMETRY |
|
| 92673. |
2-methylpent-3-enoic acid shows: |
|
Answer» OPTICAL isomerism |
|
| 92674. |
2- methylbutaneon reactionwithbrominein thepresenceofsunlightgivesmainly : |
|
Answer» 1- bromo-2-METHYLBUTANE |
|
| 92675. |
2-Methylbutane on reacting with bromine in the presene of AlBr_(3) gies mainly |
|
Answer» 2-bromo 2-methylbutane |
|
| 92676. |
2-methyl propene is isomeric with butene-1 They can be distinguished by: |
|
Answer» BAEYER's reagent |
|
| 92678. |
2-methyl propan-1-ol is obtained from 2-methyl prop-1-ene by using |
|
Answer» DIL. `H_2SO_4` |
|
| 92679. |
2- Methyl propaneonmonochlorination underphtochemical condition give : |
|
Answer» 2-choro-2-Methylpropaneas MAJORPRODUCT |
|
| 92680. |
2-methyl propanal is formed from isopropyl megnesium halide and what ? |
|
Answer» `CH_(3)CN` |
|
| 92681. |
2- methyl propane nitrile on acid hydrolysis give |
|
Answer» BUTYRIC ACID |
|
| 92682. |
2 - Methyl phenol is |
|
Answer» m - cresol |
|
| 92683. |
2-methyl phenol is |
|
Answer» m-cresol |
|
| 92684. |
2-methyl pentene-2 on ozonolysis will gives : |
|
Answer» Only propanal |
|
| 92685. |
2- methyl butane on reacting with bromine in the presence of sunlight gives mainly: |
|
Answer» 1- BROMO-3- methylbutane |
|
| 92686. |
2-methyl but-3-en-2-ol belongs to which type of alcohol? |
|
Answer» `3^@` ALCOHOL |
|
| 92687. |
2-methyl 2-propanol with Fenton's reagent gives- |
|
Answer» 1,2-methyl propene-1 |
|
| 92688. |
2-Methyl-2-propanol may be prepared by reacting methylmagnesium iodide with |
|
Answer» PROPANONE |
|
| 92689. |
2-methyl 2-pentanol is prepared from acetone and what? |
|
Answer» `C_2H_5MgI` `H_3COCH_3+CH_3CH_2CH_2MgI underset(H_3O^+)OVERSET"DRY ether "to (CH_3)_2C(OH)CH_2CH_2CH_3+MgIOH` |
|
| 92690. |
Write the relavant chemical equations : 2-Methyl--1-propene to 2-chloro-2-methyl propane |
| Answer» Solution :`CH_(3)- overset(overset(CH_(3))(|))(C )=CH_(2) overset(HCl)rarr CH_(3)- UNDERSET(underset(Cl)(|))overset(overset(CH_(3))(|))(C )-CH_(3)` | |
| 92691. |
2-methyl-1- butene on ozonolysis forms |
|
Answer» Butanone |
|
| 92692. |
2 M of 100 mL Na_2 SO_4 is mixed with 3 M of 100 mL NaCl solution and 1 M of 200 mL CaCl_2 solution . Then the ratio of the concentration of cation and anion is |
|
Answer» `1:1` |
|
| 92693. |
2-Methyl-1- bromopropane reacts with alcoholic KCN and the product formed is treated with alkaline H_(2)O_(2) to give A. The structure of A is : |
|
Answer» `CH_(3)-UNDERSET(CH_(3))underset(|)(CH)-overset(O)overset(||)(C)-NH_(2)` |
|
| 92694. |
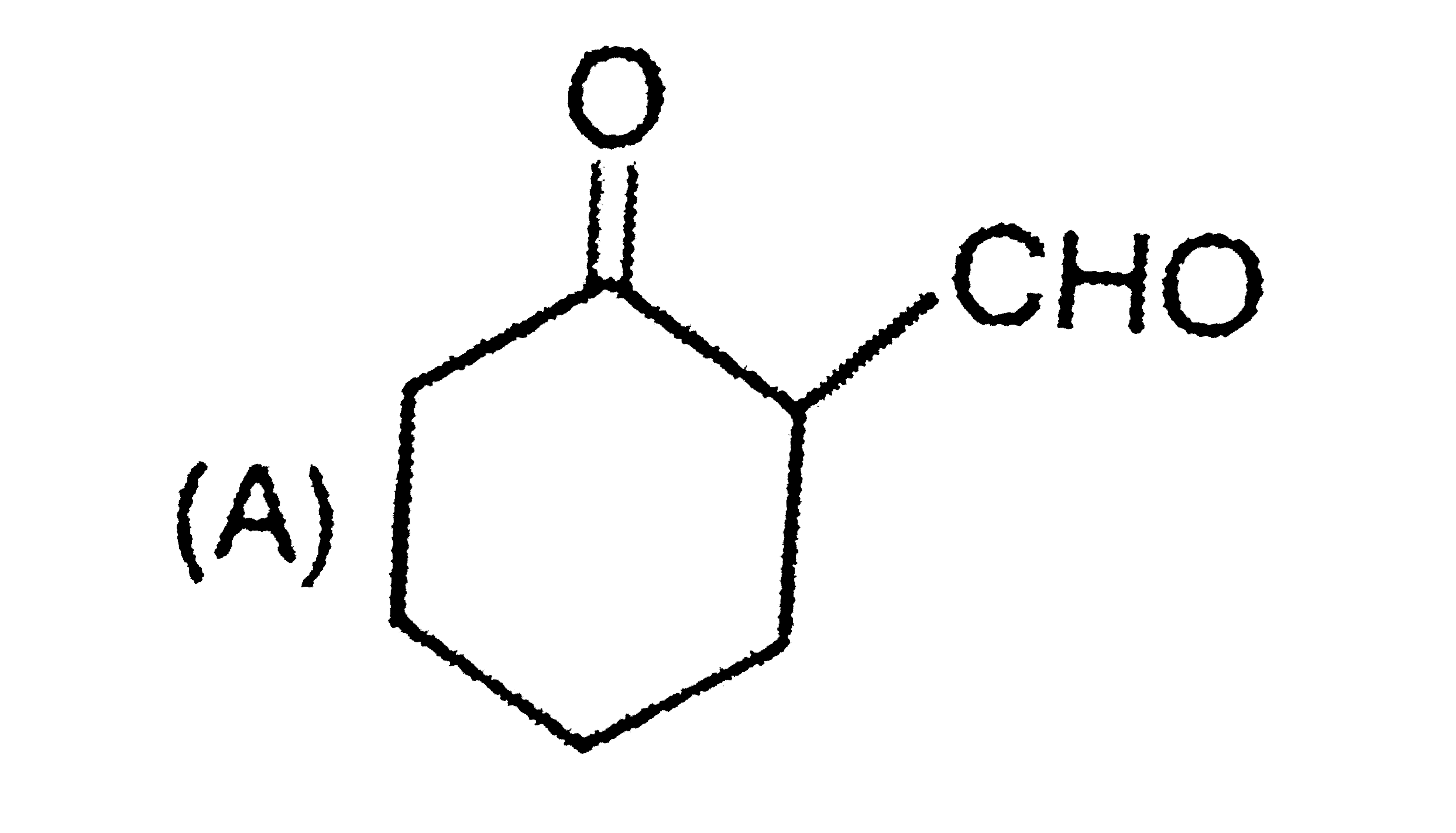
2-Methychlcyclohexanone is allowed to react with matachloroperbenzoic acid. The major product in the reaction is |
|
Answer»

|
|
| 92695. |
2 litres of ammonia at 13^(@) and 0.90atmospheric pressure is neutralised by 134 mL of H_(2)SO_(4) solution .Find the normality of the acid . |
| Answer» SOLUTION :`0.57` N | |
| 92696. |
2-Hexyne gives trans-2- Hexene on treatment with |
|
Answer» `Pt//H_2` 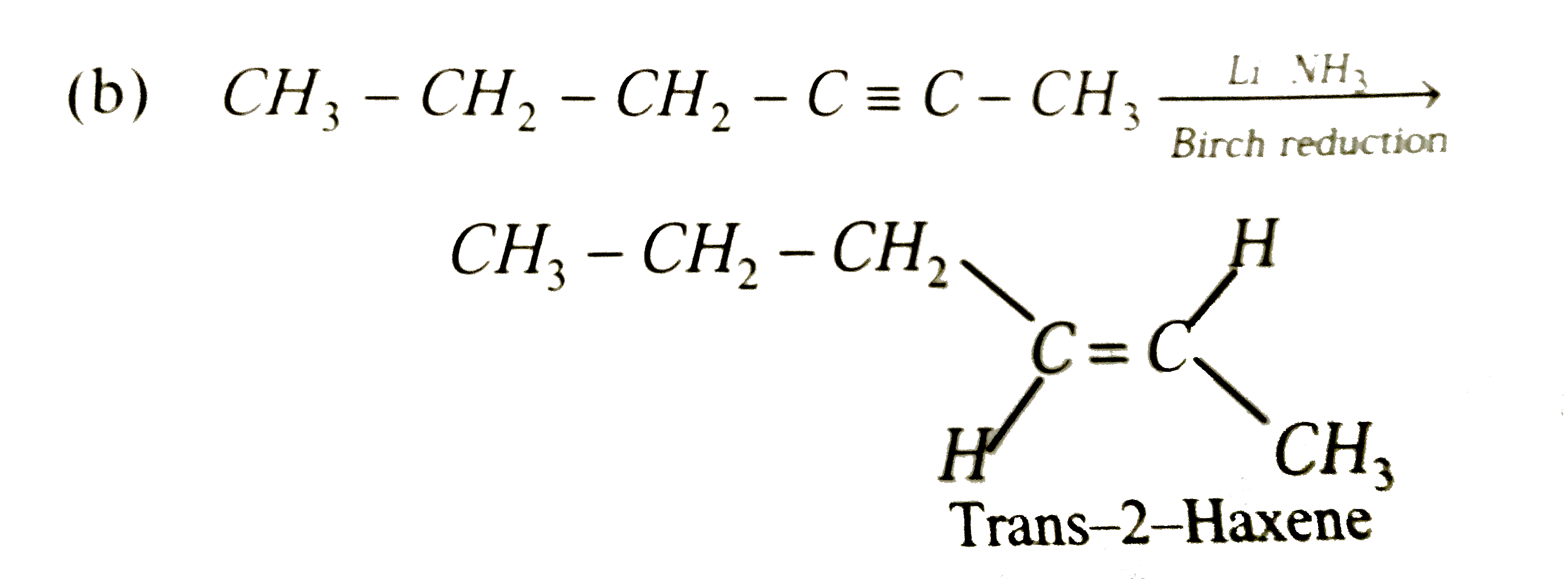
|
|
| 92697. |
1-Hexanol or Hexane which has has higher bolling points ? |
| Answer» SOLUTION :2-Hexanol | |
| 92698. |
2^@ gt 3^@ gt 1^@ |
|
Answer» Fescher-Speier esterification |
|
| 92699. |
2 g of brass containing Cu and Zn only reacts with 3M HNO_(3) solution. Following are the reactions taking place Cu(s) + HNO_(3) (aq) to Cu^(2+) (aq) +NO_(2)(g) + H_(2)O(I)Zn(s) + H^(+)(aq) + NO_(3)^(-)(aq) to NH_(4)^(+) + Zn^(2+)(aq) + H_(2)O(l)The liberated NO_(2)(g) was found to be 1.04 L at 25^(@)C and 1 atm [Cu=63.5 , Zn=65.4]The volume of HNO_(3) consumed during the reaction with brass is |
|
Answer» 9.52 ml |
|
| 92700. |
2 g of benzoic acid (C_6H_5COOH) dissolved in 25 g of benzene shows a depression in freezing point equal to 1.62 K. Molal depression constant for benzene is 4.9 K kg "mol"^(-1). What is the percentage association of acid if it forms dimer in solution ? |
|
Answer» Solution :The data provided is listed below : `W_B = 2g, K_f = 4.9 K kg "mol"^(-1) , W_A = 25 g, DELTA T_f = 1.62 K` Applying the following relation : ` Delta T_f = m xx K_f = (W_B)/(M_B) xx (1000)/(W_A) xx K_f` Substituting the values, we get `1.62 = (2)/(M_B) xx 1000/25 xx 4.9` `M_B = (2000 xx 4.9)/(25 xx 1.62) = 241.98 g"mol"^(-1)` `underset(1 -x)underset(1)(2C_6H_5COOH) iff underset(x//2)underset(0)((C_6H_5COOH)_2)` Total number of particles at equilibrium = 1 - x + x/2 = 1 - x/2 `i = (1 - x//2)/(1) = 1 - x//2` i = Calculated molecular MASS / Observed molecular mass ` =(122)/(241.98)` Thus `(122)/(241.98) = 1 - x/2` `x/2 = 1 - (122)/(241.98) = (241.98 - 122)/(241.98) = (119.98)/(241.98) = 0.4958` ` x = 0.9916 = 99.16%` The degree of association of BENZOIC ACID in benzene is 99.16%. |
|