Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 92401. |
2CHCl_3 +O_2 overset(X)rarr 2COCl_2+2HCl in the above reaction X stands for : |
|
Answer» An oxidant |
|
| 92402. |
2CH_3XtoCH_3-CH_3 What is the correct combination for the reaction: |
|
Answer» <P>(III)(i)(S) This is Wurtz REACTION |
|
| 92403. |
2CH_(3)OH+ 2Na to X + H_((2)(g)) What is X? |
|
Answer» Sodium methoxide |
|
| 92404. |
2CH_(3)CH_(2)OH underset(443K)overset( H_(2)SO_(4))to X What is X? |
|
Answer» Ethene |
|
| 92405. |
2CH_(3)CH_(2)Cl+2Na overset(" Dry ")underset(" Ether ")rarr Z+2NaCl.The (Z) in the reaction is ….. |
|
Answer» ETHANE |
|
| 92406. |
2CH_(3)CH_(2)OH + Mg to X+ H_(2(g)) What is X ? |
|
Answer» Magnesium methoxide |
|
| 92407. |
2CH_(3)CHOoverset(Al(OC_(2)H_(5))_(3))toCH_(3)COOCH_(2)CH_(3) This reaction is called: |
|
Answer» CANNIZZARO's REACTION |
|
| 92408. |
2CH_(3)-overset(O)overset(||)C-OC_(2)H_(6)overset(C_(2)H_(5)ON_(a))to A A is formed by Claisen condensation. Which is/are true about A? |
|
Answer» A FORMS oximes |
|
| 92409. |
2CaSO_4 (s) hArr 2CaO(s)+2SO_(2)(g)+O_2(g), DeltaHgt0 Above equilibrium is establised by taking some amount of CaSO_4(s) in a closed container at 1600 K. Then which of the following may be correct options ? |
|
Answer» Moles of CaO(s) will increase with the increase in temperature (B)With the increase of decrease of volume partial pressure of the gases will remain same. (C )Due to the addition of inert gases at constant pressure reaction will proceed in the direct in which more number of gaseous moles are formed. |
|
| 92410. |
2C_(6)H_(5)CHOoverset(NaOH)rarrC_(6)H_(5)CH_(2)OH+C_(6)H_(5)COONa The same reaction can take place with which of the following aldehydes |
|
Answer» `CH_(3)CHO` 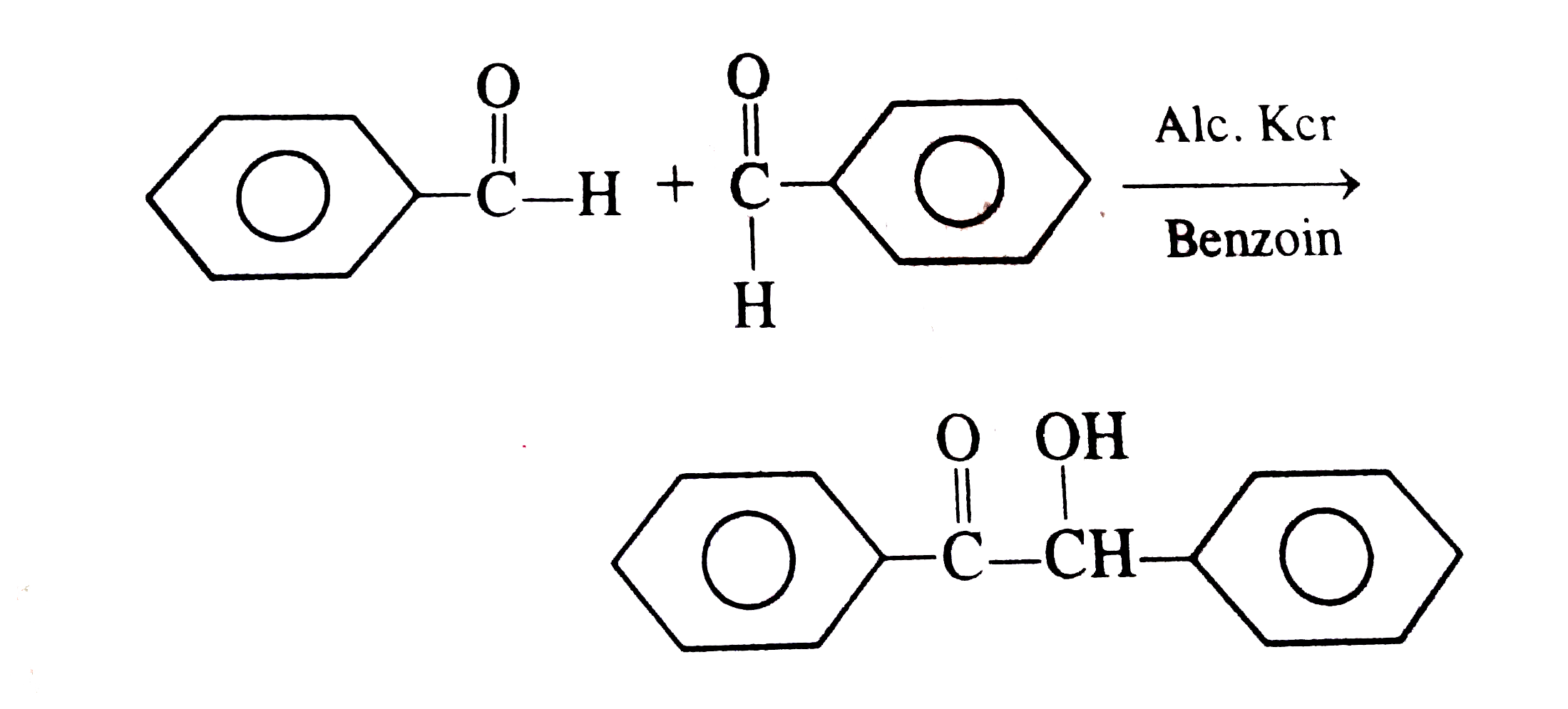
|
|
| 92411. |
2C_6H_5I underset(Delta)overset(Cu)(to)C_6H_5 - C_6H_5 + Cu_2I_2The reaction is known as |
|
Answer» fittig reaction |
|
| 92412. |
2C_6H_5CHO underset(H_2O)overset(OH^-)to C_6H_5CH_2OH + C_6H_5COO^- Which of the following statements are correct regarding the above reduction of benzaldehyde to benzyl alcohol? (i)One hydrogen is coming from H_2O as H^+ and another from C_6H_5CHO as H^- (ii)One hydrogen is coming from H_2O as H^- and another from C_6H_5CHO as H^+ (iii) One hydrogen from H_2O and another from C_6H_5CHO, both in the form of H^- (iv) The reductionis an example of disproportionation reaction |
|
Answer» (i),(ii) and (iii) |
|
| 92413. |
2C_(2)H_(5)OH overset(X)to B overset(Y)to C_(2)H_(4) +H_(2)O. Then X and Y are respectively |
|
Answer» `X = Al_(2)O_(3)` at `260^(@)C`, `Y = AICI_(3)` |
|
| 92414. |
2C_2H_5I + 2Na overset(ether)rarr C_2H_5 - C_2H_5+2NaI |
|
Answer» CLEMMENSEN reduction |
|
| 92415. |
2Br^(-)+X_(2) toBr_(2)+2X^(-). In thisreaction X_(2) is |
|
Answer» `Cl_(2)`<BR>`Br_(2)` |
|
| 92417. |
2Ag^(+) + C_(6)H_(12) O_(6) + H_(2)O rightarrow 2Ag(s) + C6H_(12)O_(7) + 2H^(+) Find ln K of this reaction. |
|
Answer» `66.13` `0.8-0.05=(0.0591)/(2xx2.303) lnK` `THEREFORE lnK=((0.8-0.05)xx2xx2xx2.303)/(0.0591)=58.45` |
|
| 92418. |
2A(g) + B(g) overset(K_(eq)=2×x10^(9))hArr3C(g) Initially only A & B are present in the container & moles of A and B at t = 0 are 4 & 2 respectively. If the concentration of A at equilibrium is p x× 10-q then find p + q - 2 Given : Volume of container is 100 lit. |
|
Answer» |
|
| 92419. |
2AtoB+C would be a zero order reaction when rate of reaction |
|
Answer» is DIRECTLY PROPORTIONAL [A] |
|
| 92420. |
2A rarr B+C. It would be a zero order reaction when. |
|
Answer» The RATE of reaction is PROPORTIONAL to square of CONC. of A |
|
| 92421. |
2A+BtoA_(2)B rate of reaction with respect to A is 3.9xx10^(-9) so calculate the rate for using of B and formation of A_(2)B |
| Answer» SOLUTION :`7.8xx10^(-9) mol L^(-1) s^(-1),r_(AV)=(Delta[B])/(Deltat)=(Delta[A_(2)B])/(Deltat)` | |
| 92422. |
2A + 3B to 4Cis a complex reaction with rate law, r=k[A]^(0) [B]^(1). if initial conc. Of A is 'a' and that of B is 'b' then what must be the ratio of b/a so that half life of A becomes equal to half life of B? Give your answer by multiplying b/a with 10. |
|
Answer» |
|
| 92423. |
{:(2A +3B to 4C + D),(""darr),("(excess)"),(3C to 2E):} If in the above reaction 6 moles of E are produced , find moles of 'A' initially taken. |
|
Answer» `4.5` MOLE |
|
| 92424. |
29.9% (w/w) HCl stock solution has a density of "1.25 g mL"^(-1). The molecular weight of HCl is "6.5 g mol"^(-1). The volume (mL) of stock solution required to prepare a 200 mL solution of 0.4 M HCl is |
|
Answer» SOLUTION :Stock solution of HCl `=29.2%(w//w)` Thus, 29.2 G of HCl are present in 100 g of the solution As density of the solution `="1.25 g mL"^(-1)`, `"volume of 100 g of the solution "=(100)/(1.25)"mL"` As molar mass of HCl `="36.5 g mol"^(-1)`, molarity of the solution `=(29.2)/(36.5)xx(1.25)/(100)xx1000=10M` `UNDERSET("(stock solution)")(M_(1)V_(1))=underset("(solution required)")((M_(2)V_(2)))` `10xxV_(1)=0.4xx200"or"V_(1)="8 mL"` |
|
| 92425. |
29.5 mg of an organic compound containing nitrogen was digested according to Kjeldahl's method and the evolved ammonia was absorbed in 20mL of 0.1M HCL solution. The excess of the acid required 15 mL of 0.1 M NaOH solution for complete neutralization. The percentage of nitrogen in the compound is: |
|
Answer» `29.5` `% N = (1.4 N_(x)V_(x))/(" mass of organic COMPOUND taken")` `%N = (1.4 xx 0.5)/(29.5 MG)` `%N = (1.4 xx 0.5)/(29.5 g) xx 1000 = 23.72` |
|
| 92426. |
29.5mg of an organic compound containing nitrogen was digested according to Kjeldahl's method and the evolved ammonia was absorbed in 20mL of 0.1M HCl solution. The excess of for complete neutralization. The percentage of nitrogen in the compound is |
|
Answer» `23.7` `0.1xxV=0.1xx15` `V_(HCl)=15ml` `:.` Volume of `HCl` USED by `NH_(3)` `=20-15=5ml` `%` of `N=(1.4NV)/(W)` `N=` Normality of acid used `V=` Volume of acid used `W=` wt. of organic compound For `HCl`, normality `=` Molarity ( `:.` Basicity is `1`) `%` of `N=(1.4xx0.1xx5)/(29.5xx10^(-3))=23.73%` |
|
| 92427. |
29.5 mg of an organic compound containing nitrogen was digested according to Kjeldahl method and the evolved ammonia was absorbed in 20 mL of 0.1 M HCI solution. The excess of the acid required 15 mL of 0.1 M NaOH solution for complete neutralisation. The percentage of nitrogen in the compound is |
|
Answer» `59.0` |
|
| 92428. |
Define molality. 29.25 gms of NaCl are present in529.25 gms of solution . Find out the molality . |
|
Answer» SOLUTION :`MOLALITY (M) = W/"mol.wt" XX 1000 /(w"in"g)` ` = 29.25/58.5 xx 1000/(529.25 - 29.25) = 1` |
|
| 92429. |
29.2% (W//W) HCl stock solutionhas a density of 1.25g mL^(-1) . The molecular weight of HCl is 36.5"mol"^(-1) . The volume (mL) of stock solution reuired to prepare a 200 mL solution of 0.4 M HCl is |
| Answer» | |
| 92430. |
28g of iron displace 3.2 g of copper from a solution of copper sulphate. If the equivalent mass of iron is 28, the equivalent mass of copper will be: |
|
Answer» 16 |
|
| 92431. |
2.84 g of methyl iodinewascompetelyconvertedintomethylmagnesiumiodineandtheproductwasdecomposedby excess of ethanol ,thevolumethegaseous hydrocarbion producedat NTPwill be : |
|
Answer» 22.4 LITRE |
|
| 92432. |
2.8 xx 10^(-2) kgofnitrogen isexpanded isothermallyandreversiblyat 300 kfrom15.15 xx 10^(5) Nm^(-2)when theworkdone isfoundto be -17. 33 kJ.Findthe finalpressure . |
|
Answer» <P> Temperature= T =300 K Workobtainedin expansion`=W_(max)=- 17 .33 kJ=-17330 J` Initialpressure`=P_(1)= 15. 15 xx 10^(5) NM^(-2)= 1.515 xx 10^(6)Nm^(-2)` Molar mass ofnitrogen`(N_(2))= M_(N_(2)) = 28xx 10^(-3)kg mol^(-1)` Finalpressure `= P_(2) = ?` Numberof MOLESOF `N_(2)= n. (W)/(M_(N_(2)))= (2.8 xx 10^(-2))/(28xx 10^(-3)) = 1 mol` `W_(max)= -2.303xx nRTlog _(10).(P_(1))/(P_(2))` `17330 = 2.303xx 1 xx 8.314xx 300xx log _(10). (1.515 xx 10^(6))/(P_(2))` `:. (17330)/(2.303 xx 1xx 8.314 xx 300)= [ log_(10) 1.515 xx 10^(6) - log_(10) P_(2)]` `3.017 =6 . 1804 -log _(10) P_(2)` `:. log_(10) P_(2) = 6.1804- 3.017 =3 . 1634` `:. P_(2)= `Antilog3.1634 `=1456.8 Nm^(-2)` |
|
| 92433. |
28 g of N_(20 and 6g of H_(2) were keip at 40^(@)C in 1 litre vesscel the equilibrium mixture contained 24.54g of NH_(3). The approximate value of K_(c)for the above reaction can be (in "mole" ^(-2) "litre"^(2) |
|
Answer» 75 `{:("Initial conc.",1,3,0),("at equlibrium",1-0.81,3-2.43,1.62),(,0.19,0.57,):}` No. of MOLES of `N_(2)=28/28=3` mole No. of moles of `H_(2)=6/2=3` moles `K_(c)=([NH_(3)]^(2))/([N_(2)][H_(2)]^(3))=([1.62]^(2))/([0.19][0.57]^(3))=75` |
|
| 92434. |
2.8 g of N_(2) , 0.40 g of H_(2) and 6.4g of O_(2) are placed in a container of 1.0 L capacity at 27^(@)C. The total pressure in the container is : |
|
Answer» 6.12 ATM `p = ( N RT )/( V )` or `p = ( 0.5 xx 0.082 xx 300)/( 1)` `= 12.3 atm` |
|
| 92435. |
28 g N2 and 6.0 g of H_(2)are heated over catalyst in a closed one litre flask of 450 °C. The entire equilibrium mixture required 500 mL of 1.0 M H_(2)SO_(4)for neutralisation. Calculate the value of Kc in L^(2) "mol"^(2)for the given reaction. N_(2)(g) + 3H_(2)(g) to 2NH_(3)(g) |
|
Answer» Moles of `H_(2)SO_(4)` required `=(500 xx 1)/1000 =0.5` Moles of `NH_(3)` neutralised by `H_(2)SO_(4)=1.0` `2NH_(3) + H_(2)SO_(4) to (NH_(4))_(2)SO_(4)` Hence 1 mole of `NH_(3)` by the reaction between `N_(2)` and `H_(2)` `{:(N_(2),+,3H_(2), |
|
| 92436. |
28 g N_2 and 6 g H_2 were mixed .At equilibrium 17 g NH_3 was formed. The weight of N_2 and H_2 of equilibrium are respectively: |
|
Answer» 11 G zero |
|
| 92437. |
2.79g of an organic compound when heated in Carius tube with conc.HNO_(3) and H_(3)PO_(4) formed converted into MgNH_(4)*PO_(4) ppt. The ppt. on heating gave 1.332 g of Mg_(2)P_(2)O_(7) . The percentage of P in the compound is |
|
Answer» `23.3%` |
|
| 92438. |
27.75 g of CaCl_2, dissolved in 250 ml of solutions. Find molarity of chloride ions in this solution. |
|
Answer» Solution :`CaCI_2 hArrCa^(2) +2CI^(-)` formulaMassof `CaCI_2 = 111` Molarityof `CI^(-)` IONS`=(27.75 )/( 111xx (250 )/(1000 ) )xx2` ` =( 55 .5 XX 1000)/( 111xx 250 )` `=2` |
|
| 92439. |
2.76 g of silver carbonate on being strongly heated yields a residue weighing : |
|
Answer» 2.16 g |
|
| 92440. |
2.76 g of silver carbonate on being strongly heated yield a residue weighing |
|
Answer» 2.16 g `2xx276 GM""4xx108 gm` `:' 2xx276 gm` of `Ag_(2)CO_(3)` GIVES `4xx108` gm `:.` 1 gm of `Ag_(2)CO_(3)` gives `=(4xx108)/(2xx276)` `:.` 2.76 gm of `Ag_(2)CO_(3)` gives `(4xx108xx2.76)/(2xx276)=2.16` gm |
|
| 92441. |
27.6 g of K_(2) CO_(3)was treated by a series of reagents so as to convert all of its carbon to K_(2) Zn_(3) [Fe (CN)_(6)]_(2)Calculate the weight of the product. |
|
Answer» Solution : `K_(2) CO_(3) underset("steps")overset("several")to K_(2) Zn_(3) [Fe (CN)_(6)]_(2)` SinceC , atoms are CONSERVE, applying POAC for C atoms, moles of CIN `K_(2) CO_(3)` = moles of C in `K_(2) Zn_(3) [ Fe (CN)_(6)]_(2)` `1 xx` molesof `K_(2) CO_(3) = 12 xx ` moles of `K_(2) Zn_(3) [Fe(CN)_(6)]_(2)` (`:. 1` mole of `K_(2) CO_(3)` contains1 mole of Cand 1 mole of `K_(2) Zn_(3) [ Fe(CN)_(6)]_(2)` contains12 moles of C) `(wt . of K_(2) CO_(3))/( mol . wt. of K_(2) CO_(3)) = 12 xx ("wt. of the product ")/("mol. wt. of product")` wt .of `K_(2) Zn_(3) [ Fe (CN)_(6)]_(2) = (27.6)/(138) xx (698)/(12) = 11.6 g` [mol . wt. of `K_(2) CO_(3)` = 138 and mol. wt. of `K_(2)Zn_(3)[Fe (CN)_(6)]_(2)=698]` |
|
| 92442. |
2.76 g of silver carbonate (at. Mass of Ag = 108) on being heated strongly yields a residue weighing |
|
Answer» 2.16 g |
|
| 92443. |
2.746 g of a compound gave on analysis 1.94 g of silver, 0.268 g of sulphur and 0.538 g of oxygen. Calculate the empirical formula of the compound (At. Masses : Ag = 108, S = 32, O = 16) |
Answer» Solution :Calculation of EMPIRICAL FORMULA  `THEREFORE"Empirical formula"=Ag_(2)SO_(4)`. |
|
| 92444. |
2.69 g of a sample of PCl_(5) was placed in a 1 litre flask and completely vaporised to a temperature of 250^(@)C. The pressure observed at this temperature was 1 atm. The possibility exists that some of PCl_(5) may have dissociated according to the equation. PCl_(5)(g) hArr PCl_(3)(g) + Cl_(2)(g). What are partial pressure of PCl_(5), PCl_(3) and Cl_(2) under these experimental conditions ? |
|
Answer» Solution :Let us first calculate the pressure supposing `PCl_(5)` does not underho dissociation. pV = nRT `p xx 1 = (2.69)/(208) xx 0.082 xx 523` [mol. WT. of `PCl_(5)` = 208, R = 0.082 lit. atm/K/mole, T - (273 + 250)K] p = 0.553 atm. But `PCl_(5)` undergoes dissociation in the following way, `{:("a (say)",0,,0),(PCl_(5) hArr,PCl_(3) ,+,Cl_(2)):} (ALPHA -= "deg. of dissociation")` Moles at eqb. : `a(1-alpha)` `therefore` TOTAL no. of moles `= a(1-alpha) + a alpha + a alpha = a(1+alpha)` `because` pressure of a gas is PROPORTIONAL to no. of moles `therefore ("moles before diss.")/("moles after diss.") = (a)/(a(1+alpha)) = (0.553)/(1)` or `alpha = 0.81`. Partial pressure of `PCl_(5) = ("moles of " PCl_(5) " at eqb.")/("total moles")xx "total pressure"` `= (a(1-alpha))/(a(1+alpha)) xx 1 = (1-0.81)/(1+0.81) = 0.104` atm. Partial pressure of `PCl_(5)` = partial pressure of `Cl_(2)` `= ("moles of " PCl_(3) " or " Cl_(2))/("total mole") xx "total pressure"` `= (a alpha)/(a(1+alpha)) xx 1 = (0.81)/(1.81) = 0.447` atm. |
|
| 92445. |
26.8gm of Na_(2)SO_(4) nH_(2)O contains 12.6gm of water. The value of 'n' is: |
|
Answer» 1 Molar mass `=(1.42+18N)` Mass of WATER`=(12.6)/(26.8)xx(142+18n)` `18n=(12.6)/(26.8)xx(142+18n)` n=7 |
|
| 92446. |
26.8 g of Na_2SO_4.xH_2O gave 12.6 g of water on heating. The value of in the compound is (M. mass of Na_2SO_4 = 142) |
|
Answer» 10 14.2 g anhy. Salt COMBINES with water = 12.6 g 142 g anhy. Salt combines with water =`12.6/14.2xx142=126` g `therefore H_2O` (MOLES) =126/18=7 |
|
| 92447. |
2.665 g of a complex with molecular formula CrCl_(3).6 H_(2)O was dissolved in water. The solution was passed through a cation exchanger such that all ionizable Cl^(-) ions passed into the solution. The solution was collected and treated with AgNO_(3) solution. The precipitate of AgCl was filtered, dried and weighed. Its mass found to be 2.87 g. Find out the structural formula of the complex and name it on IUPAC system (Atomic mass : Cr = 52, Ag = 108, Cl = 35.5, N = 14). |
|
Answer» Solution :Molar mass of the complex `CrCl_(3).6 H_(2)O=52+106.5+108=266.5 g mol^(-1)` `therefore` 2.665 g of the complex (i.e., complex reacted) = `(2.665)/(266.5)=0.01` MOLE Molar mass of AgCl = 108+35.5=143.5 g `mol^(-1)` `therefore` 2.87 g of AgCl (i.e., AgCl formed) = `(2.87)/(143.5)=0.02` mole Thus, 0.01 mole of the complex contained ionizable `Cl^(-)`=0.02 mole `therefore` 1 mole of the complex contained ionizable `Cl^(-)=(0.02)/(0.01)=2` mole This MEANS that in 1 molecule of the complex, there are `2 Cl^(-)` ions outside the coordination sphere. Remembering that coordination number of Cr is 6, the structural FORMULA of the complex will be `underset("Pentaaquachloridochromium (III) chloride monohydrate")([Cr(H_(2)O)_(5)Cl]Cl_(2).H_(2)O)` |
|
| 92448. |
2.66 g chloride of a gmetal when treated with silver nitrate solution give 2.87g of silver chloride. 3.37 g of another chloride of the same metal give 5.74 g of silver chloride when treated with silver nitrate solution. Show that the results are in agreement with a law of chemical combination. |
|
Answer» SOLUTION :Molecular mass of silver chloride `=(108+35.5)=143.5g` Mass of CHLORINE in 143.5 G of silver chloride =35.5 g So, the mass of chlorine in 2.87g of silver chlorine. `=(35.5)/(143.5)xx2.87g=0.71g`. Similarly, mass of chlorine in 5.74g of silver chloride. `=(35.5)/(143.5)=1.42g` The mass of chlorine in first metal chloride =0.71g So, the mass of metal in first metal chloride. `=(2.66-0.71)=1.95g` The mass of chlorine in second metal chloride=1.42g So, the mass of metal in second metal chloride `=(3.37-1.42)=1.95g` In both the CHLORIDES, the mass of the metal is same, but the masses of chlorine combining with the same mass of metal i.e., 1.95g are in the ratio of 0.71:1.42 or 1:2. it is a simpler atio. Thus, the results are in agreement with the law of MULTIPLE proportions. |
|
| 92449. |
26 mL of a 1 N NaCO_(3) solution is neutralised by the acids A and B in different experiments .The volumes of the acids A and B required were 10 mL and 40 mL respectively . How many volumes of A and B are to be mixed in order to prepare 1 litre of normal acid solution ? |
| Answer» SOLUTION :`179.4 ,820.6` | |
| 92450. |
26% void space in its crystal structure is obsered for A) FCC structure B) HCP structure C) CCP structure |
|
Answer» a only |
|