Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 3751. |
What is a fuel cell? |
| Answer» Solution :The galvanic cell in which the energy of combustion of fuels such as `H_(2)`, CO, `CH_(4)` etc is DIRECTLY converted into ELECTRICAL energy is CALLED the fuel cell. | |
| 3752. |
What is the difference between lowering of vapour pressure and relative lowering of vapour pressure? |
|
Answer» <P> SOLUTION :LOWERING of V.P. `=p^(@)-p_(s).`RELATIVE lowering of V.P. `=(p^(@)-p_(s))//p^(@).` |
|
| 3753. |
Which of the statements about "Denaturation" given below are correct: Statements: (a) Denaturation of proteins causes loss of secondary and tertiary structures of the protein (b) Denaturation leads to the cnversation of double strand DNA into single strand Denaturation affects primary structure which gets distorted |
|
Answer» (a),(B) and (c ) |
|
| 3754. |
What is to be done to stop corrosion of iron metal ? |
|
Answer» It should be stored in saltless water |
|
| 3755. |
Write the name and structure of one of the common initiators used in free radical addition polymerization. |
Answer» SOLUTION :BENZOYL PEROXIDE , 
|
|
| 3756. |
Which halide does not hydrolyse ? |
|
Answer» `SbCl_3` |
|
| 3757. |
What is meant by crystal field splitting energy ? On the basis of crystal field theory, write the electronic configuration of d^(4) in terms of t_(2g) and e_(g) in an octahedral field when Delta_(0)gtP |
|
Answer» Solution :The five d-orbitals in a metal ion are degenerate (of equal energy). But when ligands approach the central metal ion, they experience repulsions. The d-orbitals are SPLIT into two groups consisting of three orbitals called `t_(2G)` orbitals and two orbitals called `e_(g)` orbitals. the `e_(g)` orbitals possess greater energy than`t_(2g)` orbitals. Till there are three electrons, they will occupy three `t_(2g)` orbitals singly. If there are 4 electrons in d-orbitals i.e., `d^(4)` configuration, two CASES ARISE. If `Delta_0 GT P`, then the fourth electron will go to `t_(2g)` orbital, the electronic configuration will be `t_(2g)^(4)e_(g)^(0)` . |
|
| 3758. |
Whichone of the followingis richin liver oil,carrot , mangoand papaya ? |
| Answer» Answer :C | |
| 3759. |
What is meant by crystal field splitting energy ? On the basis of crystal field theory, write the electronic configuration of d^(4) in terms of t_(2g) and e_(g) in an octahedral field when Delta_(0)ltP |
|
Answer» Solution :The five d-orbitals in a metal ion are degenerate (of equal energy). But when ligands approach the CENTRAL metal ion, they experience repulsions. The d-orbitals are split into two groups consisting of three orbitals CALLED `t_(2g)` orbitals and two orbitals called `e_(g)` orbitals. the `e_(g)` orbitals POSSESS greater energy than`t_(2g)` orbitals. Till there are three ELECTRONS, they will occupy three `t_(2g)` orbitals singly. If there are 4 electrons in d-orbitals i.e., `d^(4)` configuration, two cases ARISE. If `Delta_(0)ltP` the fourth electron will go to `e_(g)` orbital, the electronic configuration will be `t_(2g)^(3)e_(g)^(1)` |
|
| 3760. |
Which one of the following is formed when ethanoic acid is treated with HI and Red phosphorous? |
|
Answer» ETHANE |
|
| 3761. |
Write down the IUPAC name of the complex K_(4)[Fe(CN)_(6)] |
|
Answer» Solution :FIRSTLY, the +ve PART should be named followed by the negative part in which the name of the ligand should be given in ALPHABETICAL order with-the-metal-in the negative part ending in - ate (oxidation state in parethesis) Thus, the name is Potassiumhexacyano - C-ferrate (II) HYPHEN C indicates that CN is bonded via carbon |
|
| 3762. |
Which one of the following is true in electrolytic refining ? |
|
Answer» Impure METAL is made CATHODE |
|
| 3763. |
Which of the following compounds would undergo the Cannizaro reaction ? |
|
Answer» ACETALDEHYDE |
|
| 3764. |
Whichof thefollowingcompoundgivenitrosomainewith HNO_(2) ? |
|
Answer» `1^(0)` AMINE |
|
| 3765. |
Which characteristic is not correct about H_2SO_4 |
|
Answer» REDUCING agent |
|
| 3766. |
Whatis meant bythetermpyrometallurgy? |
| Answer» SOLUTION :THEPROCESS ofextractingthemetalbyheatingthemetaloxidewithasuitablereducingagentiscalledpyrometallurgy. | |
| 3767. |
The silver glance ore on leaching with of : |
|
Answer» `Na_2(AG(CN)_2]` |
|
| 3768. |
Which of the following processes is not accompanied by increase of entropy ? |
|
Answer» DISSOLUTION of `NH_(4)CL` in water |
|
| 3769. |
Which of the following behaves as both Lewis and Bronsted base |
|
Answer» `BF_(3)` |
|
| 3770. |
Which one of the following complexes will have six isomers? |
|
Answer» `[CO(en)(NH_(3))_2CI_(2)]CI` |
|
| 3771. |
The temperature of the system increases during an: |
|
Answer» ISOTHERMAL expansion |
|
| 3772. |
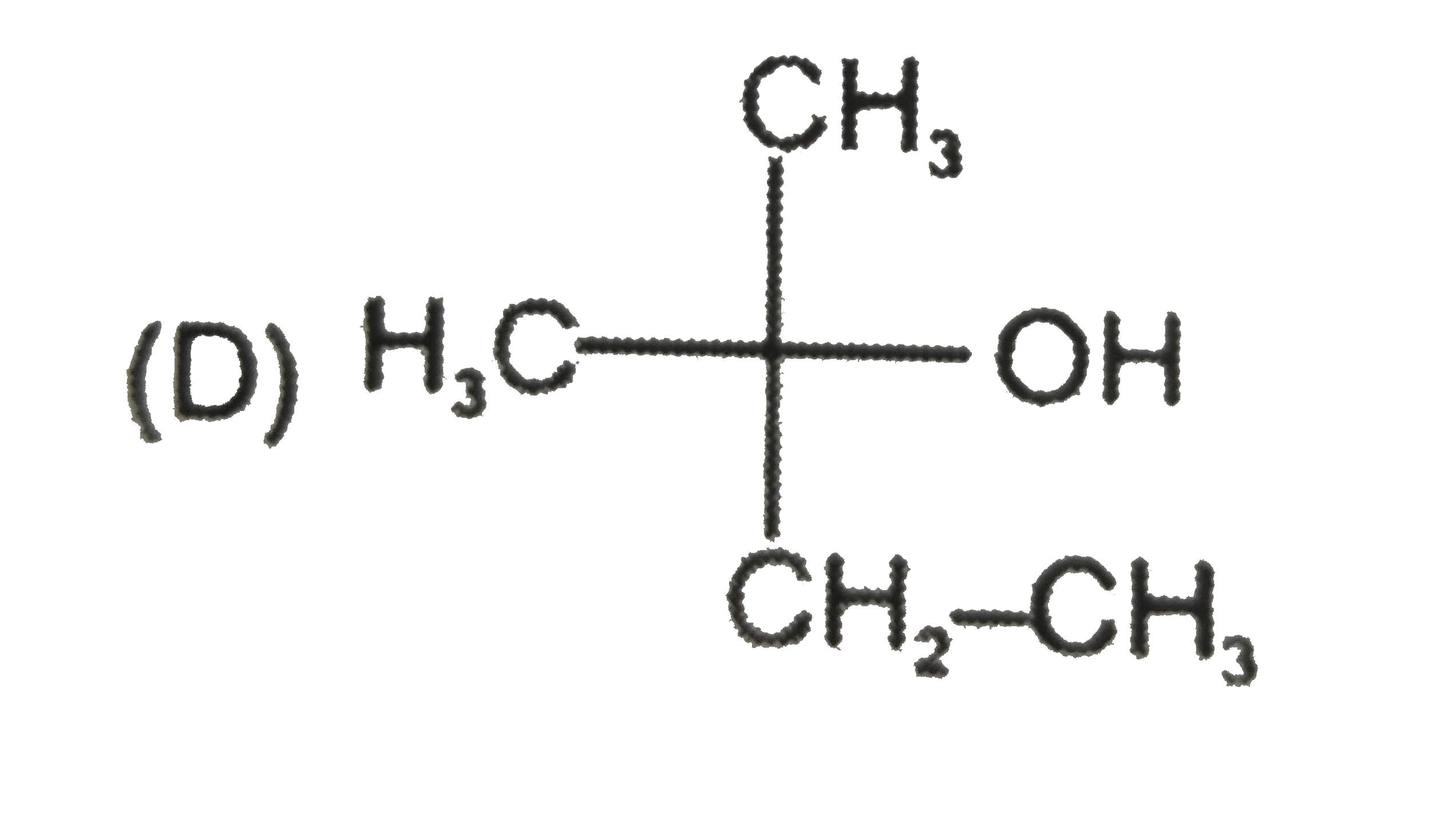
Which one of the following is a tertiary alcohol? |
|
Answer» `CH_2= CHOH` |
|
| 3773. |
Which of following species do not give borax bead test ? |
|
Answer» `CO^(+3)` |
|
| 3774. |
What is the weight of 1 mole of a solute, 0.132g of which in 29.7g of benzene, gave a freezing-point depression of 0.108^(@)C ? (K_(f) for benzene =5.12) |
|
Answer» SOLUTION :Molality `=(DeltaT_(f))/(K_(f))=(0.108)/(5.12)` ……….(EQN . 7) and ALSO molality `=(0.32)/(M)XX(1000)/(29.7)m` (moles per `1000g`) (`M=` mol.wt.or wt. of 1 mole of solute) THUS, `(0.132)/(M)xx(1000)/(29.7)=(0.108)/(5.12)` `M=211.2g"mole"^(-1)` |
|
| 3775. |
The wavelength of a moving body of mass 0.1 mg is 3.31 xx 10^(-29)m. Calculate its kinetic energy (h = 6.626 xx 10^(-34) Js) . |
| Answer» Solution :`2 XX 10^(-3) J` | |
| 3776. |
What is meant by shape selective catalysis ? |
| Answer» | |
| 3777. |
Which of the following pair of isomers can not be separated by fractional crystallisation or fractional distillation: |
|
Answer» Maleic ACID and Fumaric and |
|
| 3778. |
Which of the following complex compound exhibits geometrical isomerism? |
|
Answer» `[Fe(DMG)_(2)]` |
|
| 3779. |
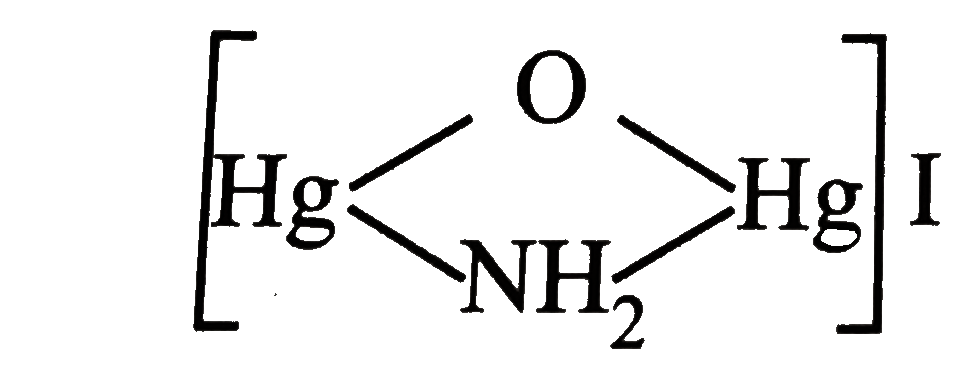
underset("in basic medium")((D))+(NH_(4))_(2)SO_(4)rarrbrown ppt (G) Hence, compound(G) is |
|
Answer» `HgI_(2)` |
|
| 3780. |
What are the various leaching processes? |
| Answer» Solution :The VARIOUS LEACHING processes are CYANIDE leaching, ammonia leaching, ALKALI leaching and ACID leaching. | |
| 3781. |
The unit of specific conductance is: |
|
Answer» `"OHM"^(-1)` |
|
| 3782. |
Which of the following systems is not correctly characterised ? |
|
Answer» CUBIC : `a=b =C,alpha=beta=gamma=90^(@)` |
|
| 3783. |
Which of the following statements is not correct for chemisorption and physisorption ? |
|
Answer» PHYSICAL ADSORPTION occurs at a low temperature and CHEMISORPTION occurs at all temperature |
|
| 3784. |
Two moles of ammonia gas are introduced into a previously evacuated 1.0" dm"^(3) vessel in which it partially dissociates at high temperature. At equilibrium 1.0 mole of ammonia remains. The equilibrium constant K_(c) for the dissociation is |
|
Answer» `27//16 ("mole dm"^(-3))^(2)` |
|
| 3785. |
Write two uses of ClO_(2). |
|
Answer» Solution :(i) `ClO_(2)` is a powerful oxidising AGENT and chlorinatingagent. Large quantities of `ClO_(2)` are used for bleaching WOOD pulp and cellulose and for purifying drinking WATER. (ii) It is an EXCELLENT bleaching agent. Its bleaching power is about 30 times HIGHER than that of `Cl_(2)` and is used for bleaching flour to make white bread. |
|
| 3786. |
Two solutions of glucose have osmotic pressures 1.5 and 2.5 atm. IL of first is mixed with 2L of second solution, the osmotic pressure of the resultant solution is |
|
Answer» 2.5+1.5 ATM |
|
| 3787. |
Which process is used for benefication of ores : |
|
Answer» PROCESS of REMOVAL of impurities |
|
| 3789. |
Write the structures of the following compounds 1,4 Dibromobut-2-ene |
| Answer» SOLUTION :`BrCH_2-CH=CH-CH_2Br` | |
| 3790. |
Two platinum electrodes were immersed in a solution of cupric sulphate and electric current passed through the solution. After some time it was found that the colour of copper sulphate disappeared with evolution of gas at the electrode. The colourless solution contains |
|
Answer» Sulphuric ACID |
|
| 3791. |
Which of the following is not the use of haloalkanes and haloarenes ? |
|
Answer» DRUGS |
|
| 3792. |
Writetheanem ofthemethodused forrefining ofthefollowing metals : (i) Titanium(ii) Germanium(iii) Copper |
|
Answer» SOLUTION :(i) Titanium - vanArkelsinceit makesavolatileiodideatlowere temperatureanddecomposesatahighertemperature. (II) GERMANIUM- Zone refiningsincetheimpuritiespresentin Gearemoresolubleinthemoltenstatethaninthesolidstateofthe metal. (iii) Copper-Electrolyticrefining. |
|
| 3793. |
Which one of the following is an example for molecular crystals ? |
|
Answer» Diamond |
|
| 3794. |
Which of the following statements regarding conc. Sulphuric acid is not correct? |
|
Answer» It is REDUCED to `SO_2` on reaction with Cu. |
|
| 3795. |
Whichof thefollowingis incorrectaboutbenzenediazoniumchloride ? |
|
Answer» These arecolourlesscrystallinesolid |
|
| 3796. |
Which of the following is the industrial method of preparation of acetaldehyde? |
|
Answer» `CH_(3)CN underset(HCl)overset(SnCl_(2))to CH_(3)CN =NH overset(H_(3)O^(+))to` |
|
| 3797. |
Which of the following is not correctly matched with its uses? |
|
Answer» Methanol : As a solvent for PAINTS VARNISHES etc. |
|
| 3798. |
Which of the following on oxidation can give a carboxylic acid in one step ? |
| Answer» Answer :D | |
| 3799. |
The structure of DNA is |
|
Answer» Linear |
|