Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 201. |
What will be product when glycine, is heated - |
|
Answer»

|
|
| 203. |
Which of the following alkene on acid catalysed hydration form propan-2-ol |
|
Answer» `CH_3CH=CH_2` `CH_3CH=CH_2 OVERSET(H_2O // H_2SO_4)to CH_3CHOHCH_3` |
|
| 204. |
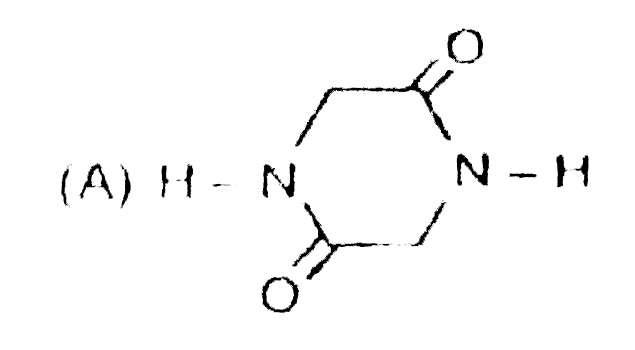
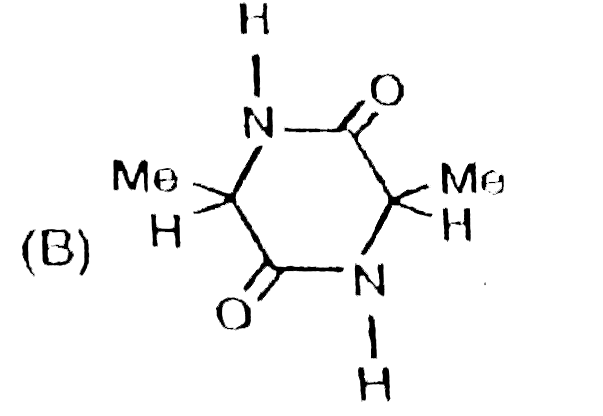
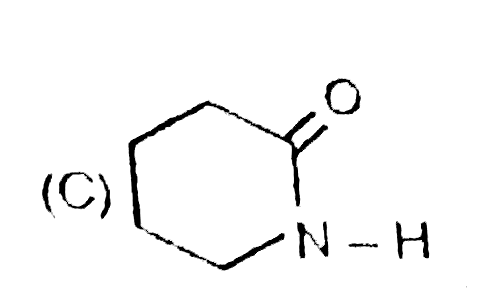
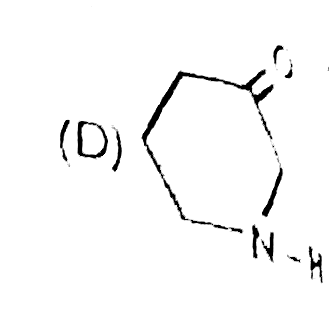
Write down the IUPAC names of A, B and C. |
|
Answer» |
|
| 205. |
Which of the following alkali metal is expected to melt if the room temperature rises to 30^(@)C ? |
|
Answer» Na |
|
| 206. |
When N_2 gas is passed through water at 293 K, how many moles of1N_2 woulddissolve in one litre water ? Assume that N_2 exert a partial pressure of 0.987 bar. K_H for N_2 at 293 K is 76.48 kilobar. |
|
Answer» Solution :By Henry.s law, MOLE fraction of `N_2 = P_(N_2)/K_H` =(0.987 bar)/(76480 bar) =`1.29xx10^(-5)`. One LITER water CONTAIN 55.5 mols of it. Let .n. be the number of mols of NITROGEN in solution , then, `X_(N_2)= n/(n+55.5)= 1.29xx10^(-5)` `therefore=1.29xx10^(-5)xx55.5=7.16xx10^(-4)`mol |
|
| 207. |
Which of the following is not a tranquilizer |
|
Answer» Luminal |
|
| 208. |
Which one of the followingis a watersolublevitamin C ? |
| Answer» Answer :C | |
| 209. |
What are the units of molar conductivity? |
| Answer» Solution :`OHM^(-1)cm^(2)mol^(-1)` or S `cm^(2)mol^(-1)`. | |
| 210. |
Which of the following elements not belongs to the d-block elements? |
|
Answer» PR |
|
| 211. |
What causes charge on sol particles ? |
|
Answer» Solution :The CAUSES of charge on sol particles are : (a) Preferential adsorption of ions from solution. (B) ELECTRON capture by sol particles during electrodispersion. (C) FORMATION of electrical double layer. |
|
| 212. |
X+NH_(3)overset(50^(@)C)toYoverset(H^(+)//H_(2)O)toH_(2)N-CH_(2)COOH Compound X is |
|
Answer» Chloroacetic acid |
|
| 213. |
Which of the following option are not in accordance with the property mentioned against them ? |
|
Answer» `F_(2) gt Cl_(2) gt Br_(2) gt I_(2)""`Oxidising power |
|
| 214. |
Which of the following statements about D(+) glucose is/are true? |
|
Answer» Naturally occurring glucose is dextrorotatory |
|
| 215. |
Write the IUPAC name of the monomer of natural rubber. |
| Answer» SOLUTION :2 - Methylbuta -1, 3 - DIENE | |
| 216. |
Which one is buffer solution |
|
Answer» `[PO_(4)^(-)] [HPO_(4)^(-)]` |
|
| 217. |
When ethyl hydrogen sulphate in heated with ethanol at 413 K, the product formed is |
|
Answer» ethyne |
|
| 218. |
Write the structures of A, B and C in the following : (i) C_(6)H_(5)-CONH_(2) overset("Br_(2)//aq.KOH)(rarr)A underset(0-5^(@)C)overset(NaNO_(2)+HCl)(rarr)B overset(KI)(rarr)C (ii) CH_(3)-Cl overset(KCN)(rarr)A overset(LiAlH_(4))(rarr)B underset(Delta)overset(CHCl_(3)+alc.KOH)(rarr)C |
|
Answer» Solution :(i) `A=underset(underset("DIAZONIUM CHLORIDE")("ANILINE"))(C_(6)H_(5)NH_(2)),""B=underset("Benzene")(C_(6)H_(5)N_(2)^(+)Cl^(-)), ""C=underset("Iodobenzene")(C_(6)H_(5)I)` (ii) `A=underset(underset("cyanide")("Methyl"))(CH_(3)CN), ""B=underset("Ethanamine")(CH_(3)CH_(2)NH_(2)), ""C=underset("Ethyl isocyanide")(CH_(3)CH_(2)NC)` |
|
| 219. |
Volume of O_(2) obtained at 2 atm & 546K, by the complete decomposition of 8.5 g NaNO_(3) is |
|
Answer» 2.24 lt. |
|
| 220. |
Write structures of the compound whose IUPAC names are as follows : 1-Phenylpropan-2-ol |
Answer» SOLUTION :
|
|
| 221. |
Which monomer is responsible for the flexibility property in PHBV ? |
|
Answer» `HO-UNDERSET(CH_(3))underset("|")"CH"-CH_(2)-CH_(2)-COOH` |
|
| 222. |
Which catalyzed biological reaction |
|
Answer» hormones |
|
| 223. |
What is the change in entropy when 2.5 mole of water is heated from 27^(@) to 87^(@) C ? Assume that the heat capacity is constant . (C_(p.m)(H_(2)O) = 4.2 J//g-k In (1.2)= 0.18) |
|
Answer» `16.6` J/K |
|
| 224. |
Which one of the following is employed as antihistamine? |
|
Answer» Chloromphenicol |
|
| 225. |
What is the name given to the linkage which holds together two monomeric units in polysaccharides? |
| Answer» SOLUTION :GLYCOSIDIC LINKAGE. | |
| 226. |
Which option is True (T) and False (F) for IUPAC name of K_(3)[Fe(CN)_(6)]? (i) potassium hexacyanido ferrate(III) (ii) tripotassium hexacyanido ferrate (iii) potassium haxacyanido ferrate (-3) tripotassium hexacyanido ferrate (III) |
|
Answer» TTTF |
|
| 227. |
Which one of the following species is stable in aqueous solution ? |
|
Answer» `CR^(2+)` `MnO_(4)^(2-)` disproptionates in nerutal or acidic solution. `3 MnO_(4)^(2-) + 4H^(+) rarr 2MnO_(4)^(2) + MnO_(2) + 2H_(2)O` |
|
| 228. |
When Cl_(2) water is added to a salt solution containing chloroform, chloroform layer turns violet. Salt contains |
|
Answer» `CL^(-)`<BR>`I^(-)` `therefore` it replaces them from their salt as `2I^(-)+Cl_(2) toI_(2)+2Cl^(-)implies`Violet vapour `2Br^(-)+Cl_(2)toBr_(2)+2Cl^(-)implies`Brown vapour |
|
| 229. |
Which of the following is an antifertility drug? |
|
Answer» Novestrol |
|
| 230. |
Which of the following used in medical field such as surgical sutures, plasma substitute? |
|
Answer» PHBV |
|
| 231. |
Which of the following sols cannot be prepared by boiling the dispersed phase with dispersion medium (water)? |
|
Answer» Egg ALBUMIN sol in water |
|
| 232. |
Which of the following is more reactive in nucleophilic substitution reaction |
|
Answer» ` (##NIK_OBJ_CHE_XII_C10_E02_017_O01.png" WIDTH="30%"> |
|
| 233. |
What happeas when an emulation is centrifuged? |
| Answer» SOLUTION :DEMULSIFICATION or BREAKS into CONSTITUENT LIQUIDS | |
| 234. |
What are homopolymers and copolymers ? |
|
Answer» SOLUTION :HOMOPOLYMERS. It is a polymer made up of only one type of monomers. Copolymers. COPOLYMER is a polymer made up of TWO or more different types of monomers. e.g. Buna-S, Nylon-66. |
|
| 235. |
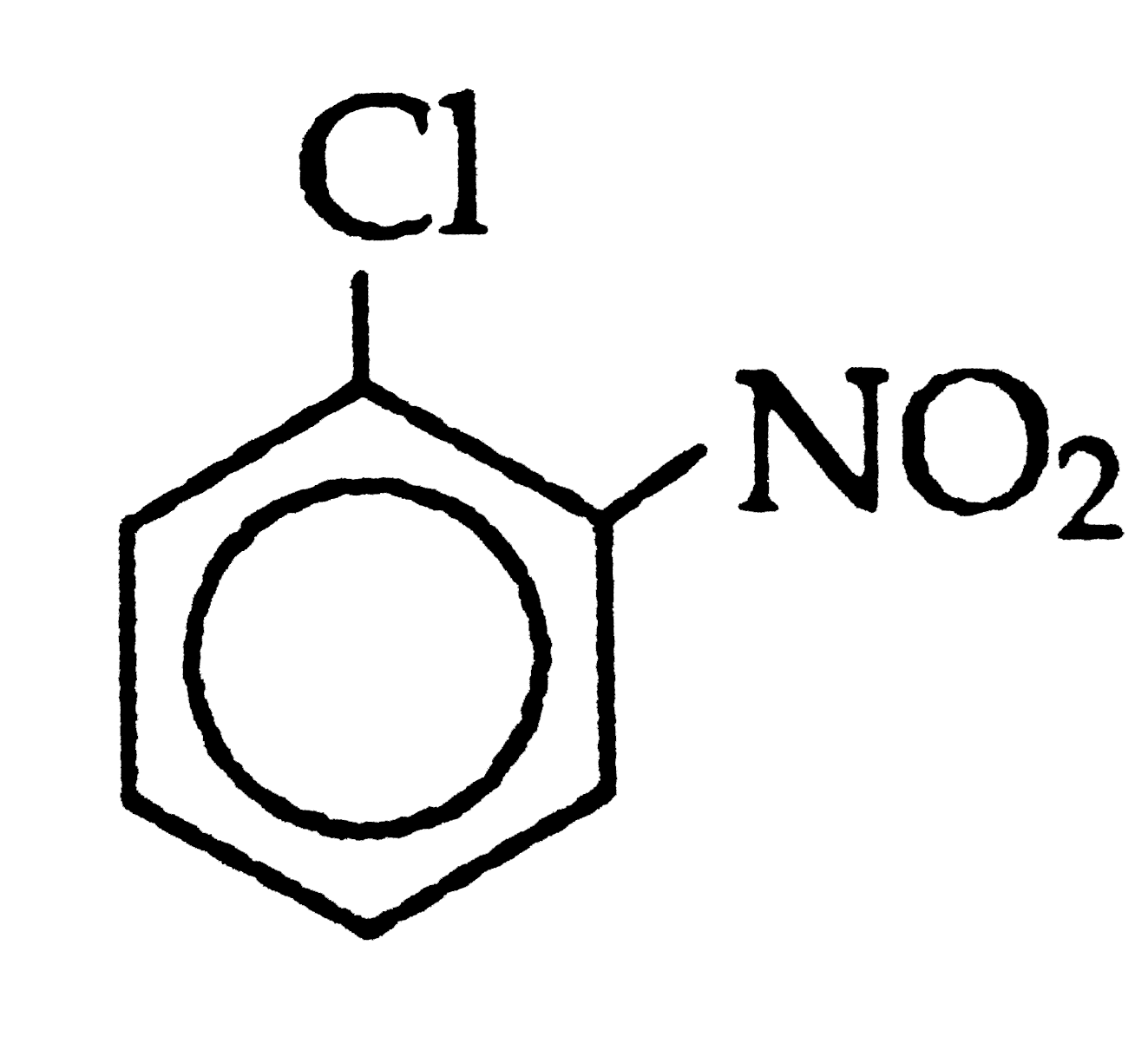
Which of the following will be the most stable diazonium salt, RN_(2)^(+)X^(-)? |
|
Answer» `CH_(3)N_(2)^(+)X^(-)` |
|
| 236. |
Which one of the following has lowest bond dissociation energy |
|
Answer» Cl-Cl  HENCE , lowest bond DISSOCIATION ENERGY is of `I_(2)`. |
|
| 237. |
Which of the following prtoducts is not formed at the anode in the hall -Heroult electrolysis process? |
|
Answer» `CO_(2)` |
|
| 239. |
Write Gibb's free energy equation and name the terms in it. |
|
Answer» SOLUTION :`G=H-TS` or `DELTA G=Delta H-T Delta S` G= Free ENERGY H= Enthalpy T= Temperature in KELVIN S= ENTROPY |
|
| 240. |
Which is not innertransition compound? |
|
Answer» TiC |
|
| 241. |
Which of the following M.Os. Containing electron/electrons has highest energy in B_(2) molecule ? |
|
Answer» `sigma 2s` |
|
| 242. |
Which of the following reactions will not yield phenol ? |
|
Answer»
|
|
| 243. |
The restriction of the biological nature and activity of proteins by heat or chemical agent is called |
|
Answer» dehydration |
|
| 244. |
Which of the following give lower abnormal molecular weight than ideal in a colligative experiment ? |
|
Answer» NaCl |
|
| 246. |
Which of the following process is not associated with recovery of the silver |
|
Answer» As a side producti in electrolytic REFINING of copper. |
|
| 248. |
Which of the following products ether, when heated with conc. H_(2)SO_(4) at 413 K? |
|
Answer» ORGANIC ACID |
|
| 249. |
Whatare LDF are HDPE ? Howare they prepared ? |
|
Answer» Solution :LDPE is LOW densitypolyethylene and HDPE is highdensity polyethylene. LDPE is preparedby polymerisationof ETHENE at temperatureof `200^(@)C` and high pressureof 100 atm in the presence of peroxideinitiator .It isa branched CHAIN polymer. HDPE is prepared bypolymerisationof ethene at a temperaturebelow `100^(@)C` and pressureless than 100 atm is the presenof Ziegler - Natta CATALYST. Itis a linear polymer . |
|
| 250. |
When KMnO_4, reacts with acidified FeSO_4 |
|
Answer» Both `FeSO_4` and `KMnO_4` are oxidised |
|