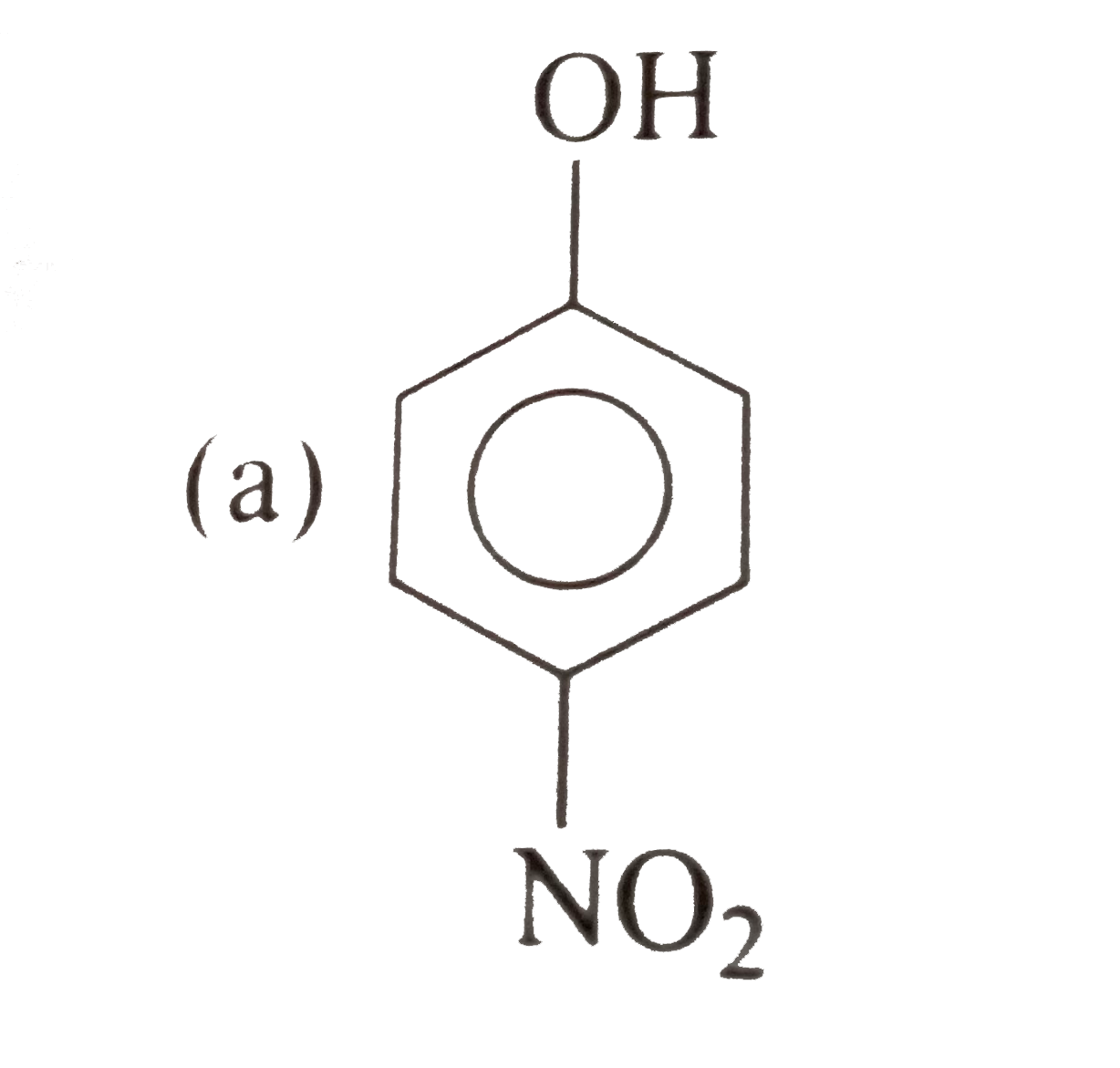Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 2101. |
When a solution of formic acid was titrated with KOH solution, the pH of the solution was 3.65 when half the acid was neutralized. Calculate K_a (HCOOH). |
| Answer» SOLUTION :`2.24 XX 10^(-4)` | |
| 2102. |
What is the number of particles per unit cell in a face centred cubic lattice ? |
|
Answer» 1 |
|
| 2103. |
The species that undergoes diproportionation in an alkaline medium are: |
|
Answer» `Cl_(4)` `Cl_(2)+2NAOH to NACL +NaOCl+H_(2)O` `2NO_(2)+2NaOH to NaNO_(2)+NaNO_(3)+H_(2)O` `MnO_(4)^(2-)` is stable in STRONG alkali solution and disproportionate into `MnO_(4)^(-) and MnO_(2)` is less basic, Acidic and Neutral medium. |
|
| 2104. |
What is crossed cannizaro reaction? Explain it. |
|
Answer» Solution :When Cannizaro reaction (AUTO redox reaction) takes place between two different aldehyde, the reaction is called as crossed cannizaro reaction. `UNDERSET("benzaldehyde")(C_6H_5CHO) + underset("formaldehyde")(HCHO) overset(NaOH)(to) underset("BENZYL alcohol")(C_6H_5CH_2OH) + underset("sodium formate")(HCOONa)` Benzaldehyde formaldehyde Benzyl alcohol sodium formate In crossed cannizaro reaction more reactive aldehyde is oxidized and LESS reactive aldehyde is reduced. |
|
| 2105. |
What is the effect of temperatuer on the solubility of a gas in a solution ? |
| Answer» SOLUTION :It DECREASES. | |
| 2106. |
When aniline is nitrated with nitrating mixutre in ice cold condition, the major product obtained is |
|
Answer» p-nitroaniline 
|
|
| 2107. |
What is meant by elementary step in a reaction? |
| Answer» SOLUTION :Each STEP of a complex REACTION is called elementary step. | |
| 2108. |
What are interstitial compounds? Why do these compounds have higher melting points than corresponding pure metals? |
|
Answer» Solution :(1)The interstitial compounds of the transition metals are those which are formed when small atoms like H, C or N are trapped INSIDE the interstitial vacant spaces in the CRYSTAL LATTICE of the metals. (2) Since metal-non-metal bonds in the interstitial compounds are stronger than metal-metal bonds in pure metals, the compounds have very high MELTING points, higher than the pure metals. |
|
| 2109. |
The structure of dibroane (B_(2)H_(6)) contains : |
|
Answer» FOUR `2C-2e` BONDS and two `3c-2e` bonds. |
|
| 2110. |
What is the oxidizing agent in the following equation ? HAsO_(2)(aq)+Sn^(2+)(aq)+H^(+)(aq)rarrAs(s)+Sn^(4+)(aq)+H_(2)Ol |
|
Answer» `HAsO_(2)(AQ)` |
|
| 2112. |
Which of the following can take part in nuclephilic as well as electrophilic substitution reactions? |
|
Answer» Ethyl BROMIDE |
|
| 2113. |
Which of the following has shortest C-C bond length ? |
|
Answer» `C_(2)H_(6)` |
|
| 2114. |
What are the limitation of VBT ? |
|
Answer» Solution :(a) It does not explain the COLOUR EXHIBITED by co-ordination compounds. (B) It does not DISTINGUISH between WEAK and strong ligands. |
|
| 2115. |
Which one of the following is not an example of complex salt ? |
|
Answer» Haemoglobin |
|
| 2116. |
Two possible stereo-structures of CH_(3)CHOH.COOH, which are optically active, are called |
|
Answer» Diastereomers |
|
| 2117. |
Which one of the following statement is true for a electrochemical cell |
|
Answer» `H_(2)` is cathode and Cu is anode |
|
| 2118. |
The shape of the complex Ag(NH_3)_2^+ is: |
|
Answer» Octahedral |
|
| 2119. |
The volume strength of 1.5 N H_2O_2 solution is : |
|
Answer» 4.8 |
|
| 2120. |
What is a protective colloid? |
|
Answer» |
|
| 2121. |
The structural formula of methyl amino methane is : |
|
Answer» `(CH_3)_2CHNH_2` |
|
| 2122. |
Two liquids X and Y form an ideal solution. At 300K, vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550 mm Hg. At the same temperature, if 1 mol of Y is further added to this solution, vapour pressure of the solution increases by 10 mm Hg. Vapour pressure (in mmHg) of X and Y in their pure states will be, respectively |
|
Answer» 200 and 300 |
|
| 2123. |
When a sulphur sol is evaporated, solidsulphur is left. On mixingwith water no colloidal sol is formed. The sulphur sol is : |
|
Answer» LYOPHILIC |
|
| 2124. |
The structure of organic compound present in a methanolic solution of ethanal which contains a trace of HCl is |
|
Answer»

|
|
| 2125. |
Which of the following arrangements the given sequence is not strictly according to the property indicated against it |
|
Answer» `HF lt HCl lt HBr lt HI` , INCREASING acidic strength `H_(2)O t H_(2)S lt H_(2)Se lt H_(2)Te`:Acidic nature (Order of `K_(a)`) `H_(2)O GT H_(2)S gt H_(2)Se gt H_(2)Te`:Order of `pK_(a)` |
|
| 2126. |
Write the IUPAC name of the comjpound given below. CH_(3)-CH_(2)-underset(CH_(3))underset(|)(C)=underset(CH_(3)OH)underset(|)(C)-OH |
| Answer» SOLUTION :3-Methylpent-2-ene-1,2-diol. | |
| 2127. |
Which one is a cross linked polymer ? |
|
Answer» BAKELITE |
|
| 2129. |
Which one is organometallic compound? |
|
Answer» `C_(2)H_(5)ONA` |
|
| 2130. |
The unsaturated hydrocarbon obtained by the reaction of a dihalogen derivative with alcoholic potash gives a red precipitate with ammoniacal cuprous chloride. The dihalogen derivative gives propanal on heating with aqueous potash. The dihalogen derivative is: |
|
Answer» 1,1–Dichloropropane |
|
| 2131. |
Which of the following molecules show p pi - p pi bonding? |
|
Answer» `p_(4)` |
|
| 2132. |
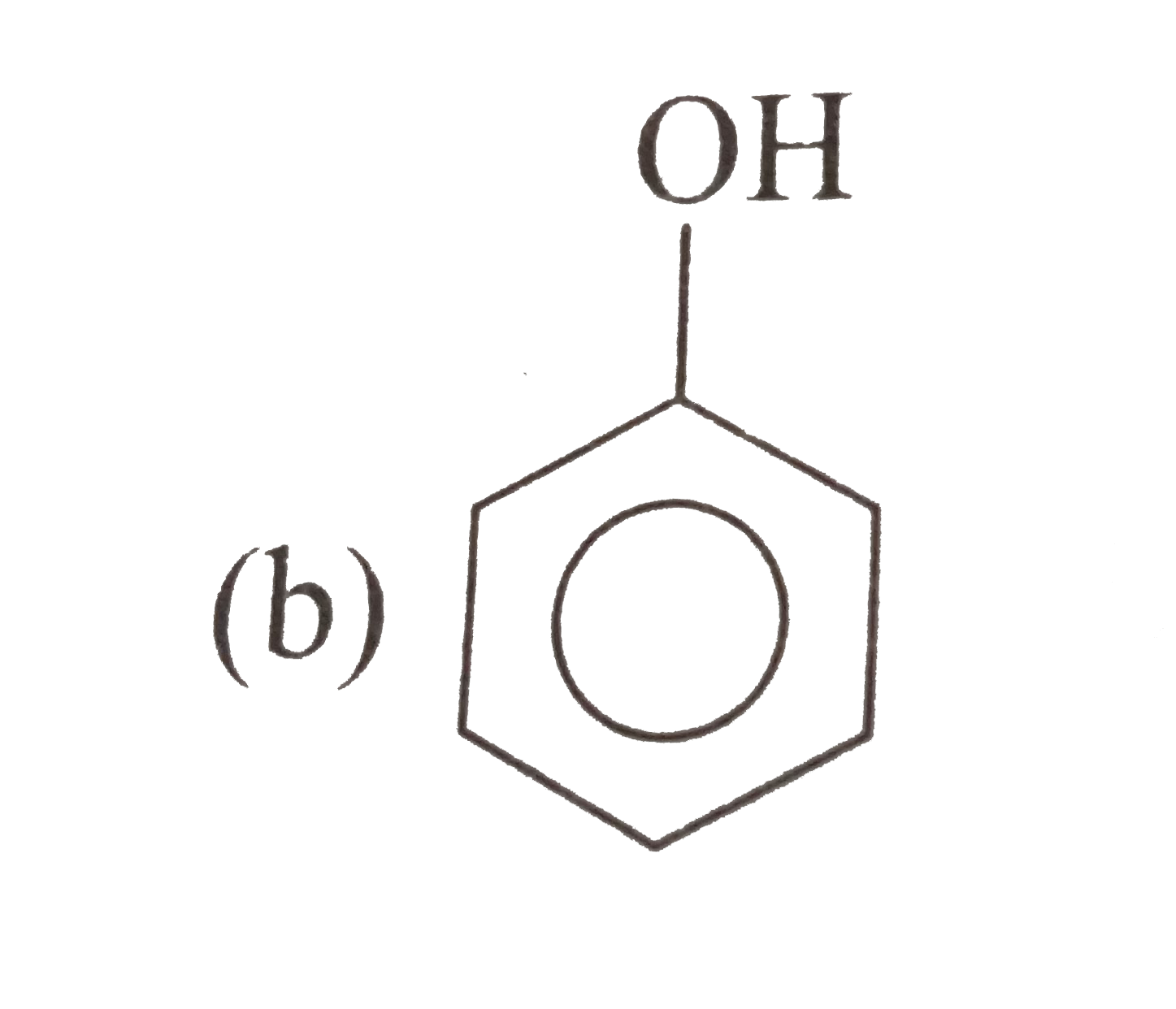
Which carboxylic acid would ou expect to be stronger A or B ? |
| Answer» Solution : The electron-withdrawing effect of the nitro GROUP WOULD help stabilize th conjugate base of B . WHEREAS the electron-donating effect of te methyl gropu in A would destablizeits conjugate base. THEREFORE B is expected to be the stronger acid. | |
| 2133. |
Which are the monomers of Terylene ? |
|
Answer» PHENOL + FORMALDEHYDE |
|
| 2134. |
Write the IUPAC names of the following coordination compound [Ni(CO)_4] |
| Answer» SOLUTION :Tetracabonylnickel (0) | |
| 2136. |
Write the oxidation number of constituent atoms in chromium peroxide. |
Answer» SOLUTION :Chromium peroxide `(CrO_(5))` is represented as  The OXIDATION NUMBER of each of the four peroxy oxygen atoms is -1 and the REMAINING oxygen atom is -2. The oxidation number of chromium atom is +6. |
|
| 2137. |
The shape of carbanion is: |
|
Answer» Linear |
|
| 2138. |
Using Gibb's free energy change, AG^@ = 57.34 kJ mol^(-1), for the reaction, X_2 Y_((s)) hArr2X^+ +Y_((aq)^3)^(2-) calculate the solubility product of X_2 Y in water at 300 K (R=8.3 JK^(-1) Mol^(-1)) |
|
Answer» `10^(-10)` |
|
| 2139. |
What type of ligand is Cl^(-), H_(2)O or NH_(3) ? |
| Answer» SOLUTION :UNIDENTATE | |
| 2140. |
Which of the followingstatements are not correct ? (I) PCl_5 is known but PBr_3 is not known. (II) In S_2F_10, sulphur atoms have octahedral geometry. (III) SF_4 is much less reactive than SF_6. (IV) Bleaching action of chlorine is permanent because it bleaches by reduction. |
|
Answer» I, III, IV |
|
| 2141. |
Which of the following compound will not liberate CO_(2) on reaction with NaOH? |
|
Answer»
|
|
| 2142. |
When 25 g of a non-volatile solute is dissolved in 100 g of water, the vapour pressure is lowered by 2.25xx10^(-1) mm, what is the molecular weight of the solute? |
|
Answer» 206 |
|
| 2143. |
Which is correct statements? |
|
Answer» STARCH is polymer of `alpha`-glucose |
|
| 2144. |
What is the oxidation number of nickel in Ni(CO)_4 ? |
|
Answer» Solution :Ni + `4XX10` = 0 The oxidation NUMBER of Ni in `Ni(CO_4)` is ZERO. |
|
| 2145. |
Which is wrong statement? |
|
Answer» The decreasing order of thermal stability is `CsOH GT RbOH gt KOH gt NaOH` |
|
| 2146. |
Two solutions of non-volatile solutes A and B are prepared. The molar mass ratio, (M_(A))/(M_(B)) = 1/3. Both are prepared as 5% solutions by weight in water. Calculate the ratio of the freezing point depressions. ((DeltaT_(f))_(A))/((DeltaT_(f))_(B)) of the solutions. If the two solutions are mixed to prepared two new solutions S_(1) and S_(2), the mixing ratio being 2:3 and 3:2 by volume for S_(1) and S_(2) respectively what would be the ratio ((DeltaT_(f))_(S_(1)))/((DeltaT_(f))_(S_(2)))? |
|
Answer» Solution :`(DeltaT_(1))A = K_(f) xx 5/(95M_(A)) xx 1000, (DeltaT_(f))_(B) = K_(f) xx 5/(95 M_(B)) xx 1000` `therefore ((DeltaT_(f))_(S_(1)))/((DeltaT_(f))_(S_(2))) = ("MOLALITY of" S_(1))/("molality of" S_(2)) = [2/M_(A) + 3/M_(B)]//[3/M_(A) + 2/M_(B)]` `=(2 +3M_(A)/M_(B))/(3+2M_(A)/M_(B)) = (2 + 3 xx 1/3)/(3 +2 xx 1/3) = 3/(3(2/3)) = 9/11` |
|
| 2147. |
Which of the following are examples of fibrous proteins? |
|
Answer» INSULIN |
|
| 2148. |
The triple bond in ethyne is made up of |
|
Answer» 3sigma bonds |
|
| 2149. |
Which of the following does not decolourize Br_(2) water |
|
Answer» `{:(CH_3),( "\"),(""C),("/"),(CH_3):} = {:(""CH_3),("/"),(C ),("\"),(""CH_3):}` |
|
| 2150. |
Which of the following configuration of ions has zero CFSE in both strong and weak ligand fields? |
| Answer» ANSWER :A | |