Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 1001. |
Which of the following is an amphoteric oxide |
|
Answer» MGO |
|
| 1002. |
When phenol on treatment with Br_(2)//H_(2)O readily gives a precipitate of 2,4,6- tribomo phenol. |
Answer» Solution :(i) PHENOL reacts with BROMINE water to GIVE a white precipitate of 2,4,6- tri bromo phenol.  (II) It readily forms tibromo derivatie since it has two-o and one-p positive FREE with respect to OH group. 
|
|
| 1003. |
Which of the following compounds is obtained when calcium acetate is dry distilled ? |
|
Answer» FORMIC acid |
|
| 1004. |
Which one of the following metallic hydroxide does not dissolve in sodium hydroxide solution? |
|
Answer» `FE(OH)_(3)` |
|
| 1005. |
Write the names of biodegradable polymers. |
| Answer» Solution :PHBV i.e. Polyhydroxy butyrate `-CO-beta-` HYDROXY valerate. DEXTRON, Nylon - 2 nylon - 6 are biodegradable polymers. | |
| 1007. |
Which of the following is a thioacid. |
|
Answer» `H_(2)S_(2)O_(4)` |
|
| 1008. |
Which of the following element do not form dpi-p pi |
|
Answer» N |
|
| 1010. |
Which of the following can exhibit cis-trans isomerism |
|
Answer» `HC -= CH` 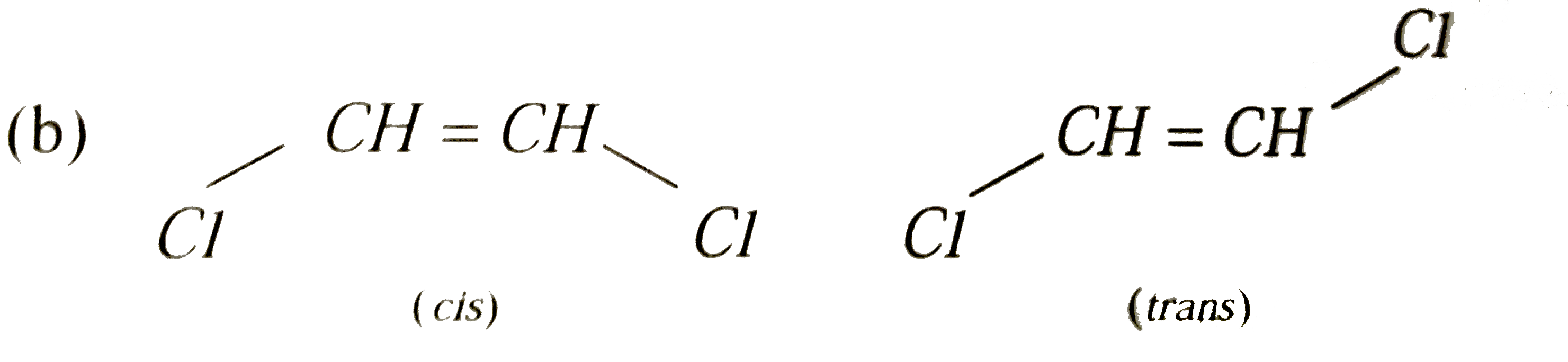
|
|
| 1011. |
When chlorine water is added to an aqueous solution of sodium halide in the presence of chloroform, a violet colouration is obtained. When more of chlorine water is added, the violet colour disappears and solution becomes colourless. This confirms that sodium haides is : |
|
Answer» chloride |
|
| 1012. |
What do you expect to happen when Red Blood Corpuscles (RBC's) are placed in (i) 1% NaCl solution (ii) 0.5% NaCl solution? |
|
Answer» Solution :(i) They will SHRINK due to PLASMOLYSIS, (ii) They will swell and may even burst. This is because RBC's are isotonic with `0.9%` NACL solution. |
|
| 1013. |
What is the wavelength of the radiation emitted producing a line in the Lyman Series when an electron falls from fourth stationary state in hydrogen atom? (R_(H)=1.1xx10^(7)m^(-1)) |
|
Answer» 96.97nm |
|
| 1014. |
Which one is the preservative in food industry? |
|
Answer» SODIUM benzoate |
|
| 1015. |
Which of the following erelationships are not correct? |
|
Answer» PH of SOLUTIO in HYDROGEN electrode`=("Electrode potential")/(0.0591)` at 298K `pH=("Electrode potential")/(0.0591)` at 298K (b) should be `E_(cell)^(@)=(0.0591)/(n)logK_(c)` (d) should be `-DeltaG^(@)=nFE_(cell)^(@)` |
|
| 1016. |
What is burnt alum ? |
| Answer» Solution :On heating to 475 K POTASH LOSES water of hydration and swells up. The swollen mass is KNOWN as burnt alum. | |
| 1017. |
What do you mean by saying that the molality of a solution is 0.2 m ? |
| Answer» SOLUTION :This means that 0.2 MOLE of the solute are DISSOLVED kg of the solvent ? | |
| 1018. |
Urea reacts with malonic ester to give: |
|
Answer» cyanuric ACID |
|
| 1019. |
Which of the following is the odd one out ? |
|
Answer» POTASSIUM ferrocyanide |
|
| 1020. |
Which one is used as a food preservative |
|
Answer» SODIUM acetate |
|
| 1021. |
When 32.25 g of ethyl chloride is subjected to dehydrohalogenation reaction, the yield of alkene formed is 50%. The mass of the product formed is (at mass of chlorine = 35.5) |
|
Answer» `14g` Molar mass of ethyl chloride `= 2xx12+5xx1+1xx35.5` `=24+9+35.5=64.5gmol^(-1)` Molar mass of ethene `=2+12+4xx1` `=28gmol^(-1)` Actual yield = 50% of 28 g = 14g 64.5g of ethyl chloride on DEHYDROHALOGENATION gives 14 g ethene `:.` 32.25 g of ethyl chloride on dehydrohalogenation will GIVE ethene = 7G |
|
| 1022. |
Wtite notes on vitamins. |
|
Answer» Solution :Vitaminis defined as an "accessory food factor which is essential for growth and healthy MAINTENANCE of body". Classification : Vitamins are broadly CLASSIFIED into TWO MAJOR groups a) the at soluble Eg : VITAMIN A, D, E and K b) water soluble Eg: vitamin B - complex and C. 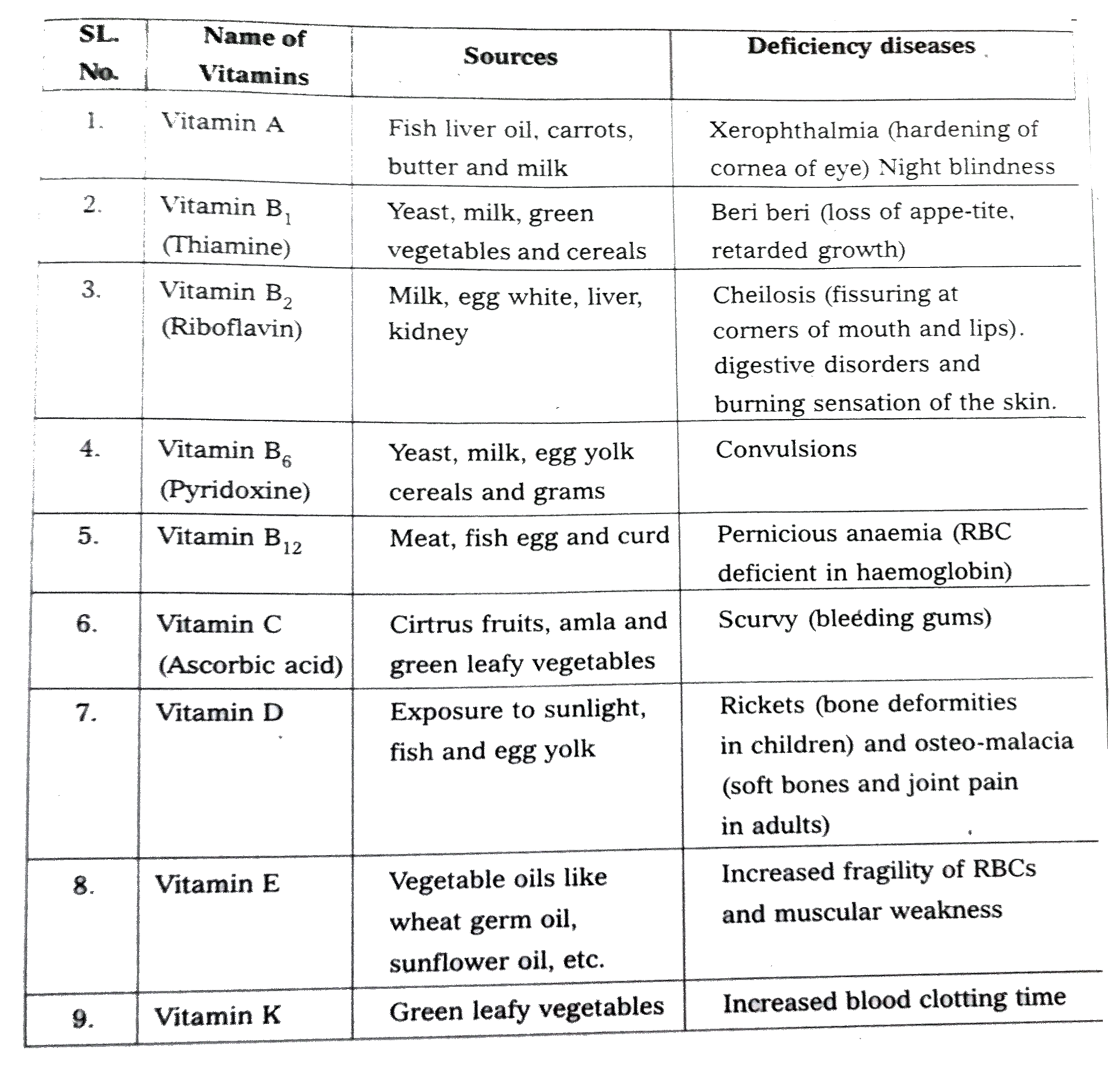
|
|
| 1024. |
When rate of forward reaction is equal and opposite to the rate of backward reaction, the state is said to be: |
|
Answer» REVERSIBLE STATE |
|
| 1025. |
The values of E_(M^(3+)//M^(2+))^(o) of metals Cr, Mn, Fe and Co are -0.41, 0.57, +0.77 and +1.97 respectively. Then whose oxidation state easily converted into +2 to +3 ? |
|
Answer» Cr `Cr^(2+) to Cr^(3+) +E^(-)E_(Cr^(3+)//Cr^(2+))^(@)=-0.41V` Minimum `Co^(2+)toCo^(3+)+e^(-) E_(Co^(3+)//Co^(2+))^(@)=1.97V` Maximum. |
|
| 1026. |
Discuss the nature of bonding and magnetic behaviour in the [FeF_(6)]^(3-) Co-ordination entities on the basis of valence bond theory. |
|
Answer» 6.92 BM |
|
| 1027. |
Vinyl derivatives undergo which type of polymerization |
|
Answer» CATIONIC POLYMERIZATION only |
|
| 1028. |
Which one of PCl_(4)^(+) and PCl_(4)^(-) is not likely to exist and why? |
|
Answer» <P> SOLUTION :`PCl_(4)^(-)`, because P has 10 ELECTRONS which cannot be accommodated in `sp^(3)` HYBRID orbitals. |
|
| 1029. |
Which of the following does not contain Mg ? |
|
Answer» MAGNETITE |
|
| 1030. |
which is used in motion picture films |
|
Answer» CELLULOSE acetate |
|
| 1031. |
The stability of +1 oxidation state among Al,Ga,In and Tl increases in the sequence |
|
Answer» GaltInltAlltTl |
|
| 1032. |
Which of the following is used in electroplating? |
|
Answer» AGCL |
|
| 1034. |
Which of the following order is incorrect :- |
|
Answer» `MCl lt MCl_(2) lt MCl_(3)`, Ionic CHARACTER<BR>`F^(-) lt Cl^(-) lt Br^(-) lt I^(-)`,Polarisibility |
|
| 1035. |
Which of the following transformations is not correct? |
|
Answer» `""_(3)^(7)Li+""_(1)^(1)Hto""_(4)^(7)Be+""_(0)^(1)N` |
|
| 1036. |
Which of the following statements is right regarding cis and trans-1,4- dibromo cyclohexane |
|
Answer» They are diastereoisomers |
|
| 1037. |
The rise in the boiling point of a solution containing 1.8 gram of glucose in 100g of a solvent is 0.1^(@)C. The molal elevation constant of the liquid is |
| Answer» Solution :`K_(B)=(DELTA T_(b))/(m)=(0.1xx100)/((1.8)/(180)xx1000)=1 K//m`. | |
| 1038. |
Write the use of aniline. |
|
Answer» SOLUTION :It is used . (i) in the preparation of dyes and dye INTERMEDIATES . (ii) in the MANUFACTURE of sulpha drugs . (iii) as a solvent in rubber INDUSTRY . |
|
| 1039. |
What happens when thionyl chloride is treated with methanol? |
|
Answer» SOLUTION :`UNDERSET("Methanol")(CH_3OH)underset("Trionyl CHLORIDE")(+SOCl_2) overse("Pyridine")tounderset("CHLORO methane")(CH_3Cl +)HCl + SO_2uarr` The above reaction follows `SN^2` mechanism in the presence of pyridine. |
|
| 1040. |
Which one is not synthetic sweeteners ? |
|
Answer» Sucrolose |
|
| 1042. |
What are interstitial compounds?Write any two characteristics of interstitial compounds. |
|
Answer» SOLUTION :The compounds FORMED by the trapping of small atoms like H,C, N etc. into the crystal LATTICE or metals. Characterstics. 1. High m.p. than the pure metal.2. Very Hard 3. Chemically inert4. Retain metaalic conductivity(any TWO). |
|
| 1043. |
Which thermodynamic property is significant in study of Ellingham diagram ? |
| Answer» Solution :Graphs are `DeltaG^(@)` vs T | |
| 1044. |
Which is the product when phenol is reacts with Br_(2), in presence of carbon disulphide at 273 - 278 K temperature ? |
|
Answer» 4- bromophenol 
|
|
| 1045. |
What is bio-degradeable polymers ? Give example. |
| Answer» SOLUTION :SYNTHETIC POLYMERS which undergo bacterial degradation in the environment, are known as BIODEGRADABLE synthetic polymers. E.g : POLY B- hydroxybutane-CO-`beta`-hydroxyl valerate (PHBV) | |
| 1046. |
The roasting reaction in the metallurgy of Cu is |
|
Answer» `CuFeS_(2) to CuO+ Fe_(2)O_(3) +SO_(2)` |
|
| 1047. |
The values of dissociation constant of [Cu(NH_(3))_(4)]^(2+) and [Co(NH_(3))_(6)]^(3+) are 1.0 xx 10^(-12) and 6.2 xx 10^(-36), respectively. Which complex would be more stable and why ? |
| Answer» Solution :`[CO(NH_(3))_(6)]^(3+)` is more STABLE because it has low VALUE of DISSOCIATION constant. It will have high stability constant. | |
| 1049. |
Which halogen shows only one oxidation number in its compounds ? |
| Answer» SOLUTION :FLUORINE i.e. -1 | |
| 1050. |
The total number of alkenes possible by dehydrobromination of 3-bromo-3-cyclopentylhexane using alcoholic KOH is |
Answer» 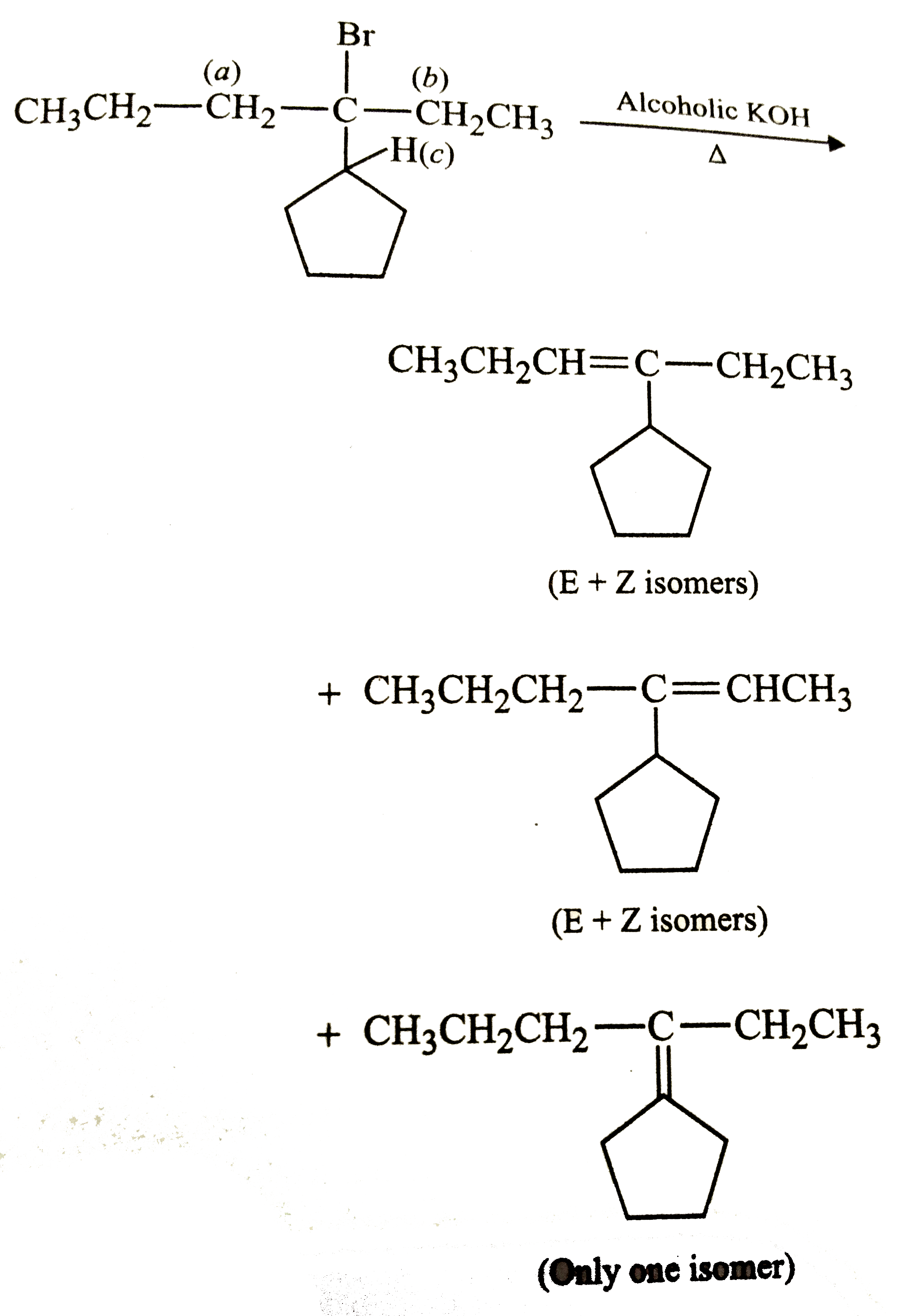
|
|