Saved Bookmarks
| 1. |
One mole of an ideal gas is taken through a cyclic process as shown in the V-T diagram. Which of the following statements is true ? |
|
Answer» The MAGNITUDE of work DONE by the gas is `RT_(0)ln2`. `W_(DA) = RT_(0) ln((2V_(0))/(V_(0))) = RT_(0) ln2` `W_(BC) = R(2T_(0))ln ((V_(0))/(2V_(0))) = -2RT_(0) ln 2` Total work done by the gas is `W = W_(AB) + W_(BC) + W_(CD) + W_(DA)` `= 0 - 2RT_(0) ln 2 + 0 + RT_(0) ln 2 = -RT_(0) ln 2` or `|W| = RT_(0) ln 2` 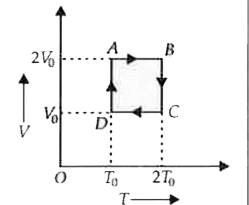
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is uniform velocity
- Derive the speed time equation by calculas method
- 9×2y - 24x^2 +16y^3.factorise?
- Give me tips for preparation of physics??
- Advantage of friction
- Derivation of equation of Newton\'s Second Law of Motion that is F=ma
- Equation of projectile?
- Unit of Permeability
- Difference between vectr and scalar
- what is centripetal force ?