Saved Bookmarks
| 1. |
Identify the substances that are oxidised and the substances that arereduced in the following reactions : (1)2H_(2)S_((g)) + SO_(2(g)) to 3S_((s)) + 2H_(2)O_((l)) |
Answer» Solution :When oxidation and reduction take PLACE simultaneously in a given CHEMICAL reaction , it is known as a REDOX reaction. 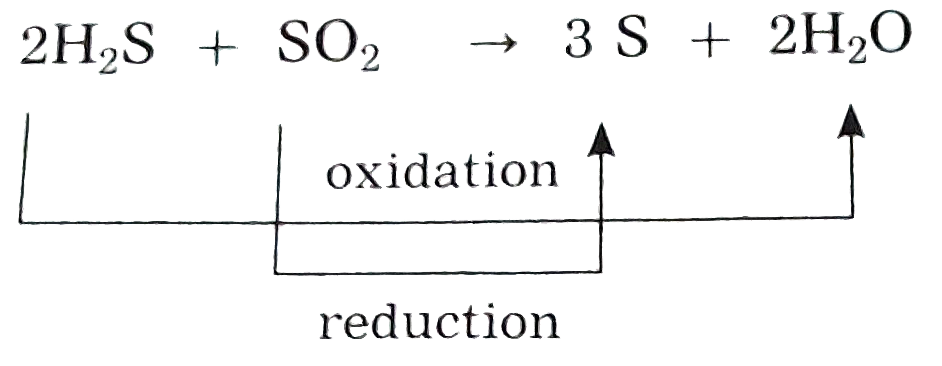 `H_(2)S` is oxidised and ` SO_(2)`is reduced. |
|
Discussion
No Comment Found
Related InterviewSolutions
- What is the reason for the twinkling of stars ?
- The change in magnetic field lines in a coil is the cause of induced electric current in it.Name the underlying phenomenon.
- Say True or False.The mass of the Earth is 6.4xx10^(6)kg.
- Whichof the follwing property of a proton can changewhileit moves freelyin a mageticfield ? (There may be more thanone correct answer).
- When an object is placed infront of a spherical mirror at a distance 30 cm, the magnification is -1. Illustrate the conclusions.
- Does the frequency of sound waes depend on the medium in which it travels?
- The relation between N (no. of molecules), P,V. & T is ...........
- What is magnification of a lens ?
- A person is said to be colour blind if he/ she has deficiency of rod shaped cells in retina of his eyes.
- "___________" prepares the 'Red List' that contains the names of endangered species from different countries.